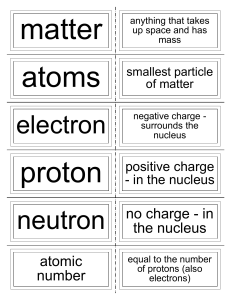
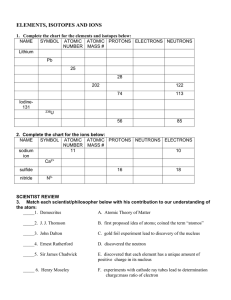
Quiz: The Atom (Open Notes)
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
Learning Standards vocab chemical basis and molecules of life 09
... sources of food and nutrition, fossil fuels). Describe at least three chemical reactions of particular importance to humans (e.g., burning of fossil fuels, photosynthesis, rusting of metals). Use a chemical equation to illustrate how the atoms in molecules are arranged before and after a reactio ...
... sources of food and nutrition, fossil fuels). Describe at least three chemical reactions of particular importance to humans (e.g., burning of fossil fuels, photosynthesis, rusting of metals). Use a chemical equation to illustrate how the atoms in molecules are arranged before and after a reactio ...
AP Chem Test 5-7 Practice Exam - mvhs
... 2. The ΔH for the exothermic solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When a 13.9-g sample of NaOH dissolves in 250.0 g of water in a coffee-cup calorimeter, the temperature change is _______. Assume that the solution has the same specific heat as liquid water, ...
... 2. The ΔH for the exothermic solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When a 13.9-g sample of NaOH dissolves in 250.0 g of water in a coffee-cup calorimeter, the temperature change is _______. Assume that the solution has the same specific heat as liquid water, ...
Understanding the Atom GN
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
CLASS TEST NAME Class IIB Date ______ 1 .Which atomic
... 9. _______________________________________ are atoms of the same element that have different numbers of neutrons. 10.The atomic number of an element tells you the number of _________________________________________. 11. An ion is a form of an atom with a different number of__________________________ ...
... 9. _______________________________________ are atoms of the same element that have different numbers of neutrons. 10.The atomic number of an element tells you the number of _________________________________________. 11. An ion is a form of an atom with a different number of__________________________ ...
1 - cloudfront.net
... All atoms of the same element have the same _____. Know Dalton’s Atomic Theory. How is the number of neutrons in the nucleus of an atom calculated? All atoms are neutral, with the number of protons equaling the ___. Isotopes of the same element have different _____. Using the periodic table, determi ...
... All atoms of the same element have the same _____. Know Dalton’s Atomic Theory. How is the number of neutrons in the nucleus of an atom calculated? All atoms are neutral, with the number of protons equaling the ___. Isotopes of the same element have different _____. Using the periodic table, determi ...
Chapter 3 Chemical Foundations
... Atomic Number, Mass Number, and Nucleus atomic number (Z) = mass number (A) = element symbol (X) = Note: mass number= Therefore …. mass number = ……. A= Z + number of neutrons ….. Number of neutrons = A-Z Note: For any given element on the periodic table: Number of protons = In order to symbolically ...
... Atomic Number, Mass Number, and Nucleus atomic number (Z) = mass number (A) = element symbol (X) = Note: mass number= Therefore …. mass number = ……. A= Z + number of neutrons ….. Number of neutrons = A-Z Note: For any given element on the periodic table: Number of protons = In order to symbolically ...
Atomic Structure
... •Even though isotopes have different amounts of neutrons they are still chemically alike since they have the same number of protons and electrons. •To find the most common isotope round the atomic mass to nearest whole number. -Ex: Carbon-12 is the most common isotope of carbon Which isotope is the ...
... •Even though isotopes have different amounts of neutrons they are still chemically alike since they have the same number of protons and electrons. •To find the most common isotope round the atomic mass to nearest whole number. -Ex: Carbon-12 is the most common isotope of carbon Which isotope is the ...
Atomic Structure Power Point
... Because of ISOTOPES ! An isotope is a form of an element that has the same number of protons, but different numbers of neutrons. The atomic mass on the periodic table reflects the average mass of all of the known isotopes of an element. Each isotope may have different characteristics. ...
... Because of ISOTOPES ! An isotope is a form of an element that has the same number of protons, but different numbers of neutrons. The atomic mass on the periodic table reflects the average mass of all of the known isotopes of an element. Each isotope may have different characteristics. ...
VOCABULARY name, date, hour: Fill in the number of each term
... ___ positively charged particle found in the nucleus of an atom ___ stable, orbiting particle of an atom with a negative charge ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ el ...
... ___ positively charged particle found in the nucleus of an atom ___ stable, orbiting particle of an atom with a negative charge ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ el ...
Periodic Table Review Key
... Would atom A gain or lose valence electrons? __lose__ Would atom B gain or lose valence electrons? __neither__ ...
... Would atom A gain or lose valence electrons? __lose__ Would atom B gain or lose valence electrons? __neither__ ...
Ch. 2 note packet
... Essentials of his theory. . . 1. An element is composed of tiny particles called atoms. All atoms of a given element show the same chemical properties. 2. Atoms of different elements have different properties. In an ordinary chemical reaction, no atom of any element disappears or is changed into an ...
... Essentials of his theory. . . 1. An element is composed of tiny particles called atoms. All atoms of a given element show the same chemical properties. 2. Atoms of different elements have different properties. In an ordinary chemical reaction, no atom of any element disappears or is changed into an ...
1.2 Atomic Theory
... The average atomic mass for magnesium found on the periodic table is a weighted average of the three isotopes: 24.31 g of Mg Radioactivity: spontaneous decay of nuclei, releasing energy and subatomic particles Radioisotopes: an unstable isotope of an element, which undergoes radioactive decay ...
... The average atomic mass for magnesium found on the periodic table is a weighted average of the three isotopes: 24.31 g of Mg Radioactivity: spontaneous decay of nuclei, releasing energy and subatomic particles Radioisotopes: an unstable isotope of an element, which undergoes radioactive decay ...
Chemistry Review: Antoine Lavoisier (1743
... are made up of atoms (which cannot be created or destroyed chemically), which make up molecules. (which can be created or destroyed chemically) Molecule (latin: molecula) means “little mass” Parts of the Atom: proton found in the nucleus (+ charge), neutron found in the nucleus (neutral) and the ele ...
... are made up of atoms (which cannot be created or destroyed chemically), which make up molecules. (which can be created or destroyed chemically) Molecule (latin: molecula) means “little mass” Parts of the Atom: proton found in the nucleus (+ charge), neutron found in the nucleus (neutral) and the ele ...
History of the Atom and Periodic Table
... had a neutral charge and it is called the neutron. His discovery made us realize isotopes existed. Isotopes are atoms of the same element with a different number of neutrons. Proved Dalton’s Atomic theory was incorrect again by showing atoms of the same element can be different. ...
... had a neutral charge and it is called the neutron. His discovery made us realize isotopes existed. Isotopes are atoms of the same element with a different number of neutrons. Proved Dalton’s Atomic theory was incorrect again by showing atoms of the same element can be different. ...
NOTES: 2.1 - Intro to Chemistry
... ATOM: smallest unit of matter that retains the physical and chemical properties of its element ● three subatomic particles: ...
... ATOM: smallest unit of matter that retains the physical and chemical properties of its element ● three subatomic particles: ...
Introduction_to_Geochemistry_Pre-Lecture_Quiz
... (a) The diameter of an atom is less than the diameter of its nucleus. (b) The relative atomic mass of an atom is the mass of an atom relative to an atom of 12C. (c) p-orbitals can contain a maximum of 10 electrons. (d) The first ionisation energy of an element is the energy input (in kg mol-1) requi ...
... (a) The diameter of an atom is less than the diameter of its nucleus. (b) The relative atomic mass of an atom is the mass of an atom relative to an atom of 12C. (c) p-orbitals can contain a maximum of 10 electrons. (d) The first ionisation energy of an element is the energy input (in kg mol-1) requi ...
Salesian High School Elements and atoms Chemistry quiz The
... a) Atoms that have the same mass number but different atomic number b) Elements that have the same mass number but different atomic numbers c) Atoms that have the same number of protons but a different number of neutrons d) Elements that have the same number of neutrons but different mass numbers ...
... a) Atoms that have the same mass number but different atomic number b) Elements that have the same mass number but different atomic numbers c) Atoms that have the same number of protons but a different number of neutrons d) Elements that have the same number of neutrons but different mass numbers ...