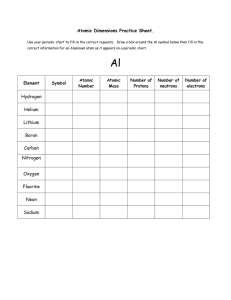
Atoms of an element can lose or gain electrons and still
... 6. In an atom, electrons can be grouped into _________ levels, each holding only a specific number of electrons. (pg. 36, P5) 7. All atoms of the same element have the same number of ________ .(pg. 37, P1) ...
... 6. In an atom, electrons can be grouped into _________ levels, each holding only a specific number of electrons. (pg. 36, P5) 7. All atoms of the same element have the same number of ________ .(pg. 37, P1) ...
Chapter 5 - Effingham County Schools
... nucleus, called the ________ ________. For example, a hydrogen atom has 1 proton so its atomic number is 1. The total number of _______ and _________ in an atom’s nucleus is called its atomic mass number. _______ are atoms of the same element that have a different number of neutrons. Ions are formed ...
... nucleus, called the ________ ________. For example, a hydrogen atom has 1 proton so its atomic number is 1. The total number of _______ and _________ in an atom’s nucleus is called its atomic mass number. _______ are atoms of the same element that have a different number of neutrons. Ions are formed ...
Ch. 4: Atoms and the Periodic Table – Study Guide
... The first person who suggested that matter was made up of atoms was the Greek philosopher Democritus. The word atom comes from the Greek word that means “unable to be divided.” Dalton’s atomic theory stated that every element was made of atoms that could not be subdivided, atoms of the same element ...
... The first person who suggested that matter was made up of atoms was the Greek philosopher Democritus. The word atom comes from the Greek word that means “unable to be divided.” Dalton’s atomic theory stated that every element was made of atoms that could not be subdivided, atoms of the same element ...
The Periodic Table
... Mass number is the count of nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
... Mass number is the count of nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
Name Date Class Period ______
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
... Name ______________________________________ Date __________________ Class Period _________ Atoms, Elements, and Compound Test Study Guide I. ...
Keypoints of Basic Atomic Structure
... Atomic Number Atomic Radius Electrons Element Isotope Neutrons Periodic Table Protons Subatomic Particles Concepts 1. Be able to describe how protons, neutrons and electrons are arranged in an atom. 2. Be able to list the charges on the subatomic particles that make up and atom, and giv ...
... Atomic Number Atomic Radius Electrons Element Isotope Neutrons Periodic Table Protons Subatomic Particles Concepts 1. Be able to describe how protons, neutrons and electrons are arranged in an atom. 2. Be able to list the charges on the subatomic particles that make up and atom, and giv ...
Atoms, Elements, Compounds File
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
Chemical reactions revision
... Elements are the building blocks of chemistry. Every element contains only one type of atom Each element contains atoms different to every other element Elements are arranged in the Periodic Table of elements. Element are arranged in the table in order of their atomic number Elements in different gr ...
... Elements are the building blocks of chemistry. Every element contains only one type of atom Each element contains atoms different to every other element Elements are arranged in the Periodic Table of elements. Element are arranged in the table in order of their atomic number Elements in different gr ...
HW-1-Ch1-Atomic-structure-W16
... 4. Calculate the binding energy per nucleon (MeV) of 56Fe isotope of mass 55.952918 amu. ( P= 1.007277 amu,; N= 1.008665 amu; e- = 5.486 x10-4 amu) ...
... 4. Calculate the binding energy per nucleon (MeV) of 56Fe isotope of mass 55.952918 amu. ( P= 1.007277 amu,; N= 1.008665 amu; e- = 5.486 x10-4 amu) ...
Study Guide Answer Key
... The mass doesn’t change in chemical reactions because the atoms only rearrange in the reaction, nothing is added or removed from the system. ...
... The mass doesn’t change in chemical reactions because the atoms only rearrange in the reaction, nothing is added or removed from the system. ...
atomic structure - IGCSE STUDY BANK
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
I can describe an atom and its components I can relate energy levels
... ○ All atoms of the same element have the same number of protons ○ The number of neutrons can vary ○ ex)Chlorine atoms have 17 protons but can have 18 or 20 neutrons. ■ There are chlorine atoms with mass #s of 35 and 37. (17+18=35, 17+20=37) ...
... ○ All atoms of the same element have the same number of protons ○ The number of neutrons can vary ○ ex)Chlorine atoms have 17 protons but can have 18 or 20 neutrons. ■ There are chlorine atoms with mass #s of 35 and 37. (17+18=35, 17+20=37) ...
are made up of
... that grouped elements accordingto their properties. They found that these properties repeated in a regular or periodic manner. This fact was used to predict properties of undiscovered elements. Reviewelectron arrangement from your textbook.In Table I, write.the maximum number of electrons that can f ...
... that grouped elements accordingto their properties. They found that these properties repeated in a regular or periodic manner. This fact was used to predict properties of undiscovered elements. Reviewelectron arrangement from your textbook.In Table I, write.the maximum number of electrons that can f ...
Chapter 3 Atoms and Elements
... Since atomic mass is equal to the sum of the number of protons and neutrons, how can you have a fractional number? How was an atomic mass value of 35.45 arrived at? Since in a “handful” of Cl there is a mixture of two isotopes in the abundances shown on the left, an average atomic mass has been defi ...
... Since atomic mass is equal to the sum of the number of protons and neutrons, how can you have a fractional number? How was an atomic mass value of 35.45 arrived at? Since in a “handful” of Cl there is a mixture of two isotopes in the abundances shown on the left, an average atomic mass has been defi ...
chapter 7 – cyu
... Geissler develop the gas discharge tube. This tube, when it has all the air pumped out of it, initially glowed blue then green at one end when under very low pressure. The green glow at the anode end was the result of the electrons being released from the cathode at the opposite end of the tube. The ...
... Geissler develop the gas discharge tube. This tube, when it has all the air pumped out of it, initially glowed blue then green at one end when under very low pressure. The green glow at the anode end was the result of the electrons being released from the cathode at the opposite end of the tube. The ...
Chapter 11 and 12-2 Review/Study Guide for Test
... 1. List the major discovery or accomplishment associated with of each of the following scientists: a. Democritus – named the atom, stated it was indivisible b. Dalton – atom is positively charged sphere - said atoms of different elements are different, atoms of the same element are the same c. Thoms ...
... 1. List the major discovery or accomplishment associated with of each of the following scientists: a. Democritus – named the atom, stated it was indivisible b. Dalton – atom is positively charged sphere - said atoms of different elements are different, atoms of the same element are the same c. Thoms ...
File
... PRACTICE: How many electrons shells would be completely filled by a neutral atom of calcium? How many electrons would be left over? ...
... PRACTICE: How many electrons shells would be completely filled by a neutral atom of calcium? How many electrons would be left over? ...
Exam 1 Review Questions
... Six elements have been found or made in such small amounts that their physical properties are unknown. ...
... Six elements have been found or made in such small amounts that their physical properties are unknown. ...
element - Mrs. Phillips` Physical Science Webpage
... • The periodic table is arranged by increasing atomic number. – During Mendeleev’s time, this arrangement left several blanks, however, the table exhibited a regularly repeating pattern, which could be used to predict the properties of elements that had not been discovered yet. – He was proven right ...
... • The periodic table is arranged by increasing atomic number. – During Mendeleev’s time, this arrangement left several blanks, however, the table exhibited a regularly repeating pattern, which could be used to predict the properties of elements that had not been discovered yet. – He was proven right ...
Atomic Structure AKS Correlation Use the modern atomic theory to
... Matter cannot be empty space, dense and its’ makecreated or center up of atoms destroyed Atoms of same element look the same Chem reactions occur when atoms are rearranged Compounds formed by 2 or more atoms Describe the basic structure of the atom as protons, neutrons and electrons in specific arra ...
... Matter cannot be empty space, dense and its’ makecreated or center up of atoms destroyed Atoms of same element look the same Chem reactions occur when atoms are rearranged Compounds formed by 2 or more atoms Describe the basic structure of the atom as protons, neutrons and electrons in specific arra ...
Atomic Structure and the Periodic Table
... • Electron cloud – cloud that surrounds the nucleus of an atom that describes the region in which an electron is most likely to be. – Example: students in a school ...
... • Electron cloud – cloud that surrounds the nucleus of an atom that describes the region in which an electron is most likely to be. – Example: students in a school ...