bohrmodelofatomclassnote0
... neutrons – these particles both weigh 1 atomic mass unit (1 amu). We round the mass number to a whole number (it’s a decimal due to isotopes – tomorrow…) The masses are scaled from a standard – C-12 which has a mass of 12 amu (more later) The atomic number tells us the number of protons in the n ...
... neutrons – these particles both weigh 1 atomic mass unit (1 amu). We round the mass number to a whole number (it’s a decimal due to isotopes – tomorrow…) The masses are scaled from a standard – C-12 which has a mass of 12 amu (more later) The atomic number tells us the number of protons in the n ...
Chapter 4
... Other Elements on the periodic table • ____________ Transition Metals - elements in Group 3 through 12. These metals are hard and shiny, good conductors of electricity, less reactive than metals in Groups 1 and 2. ________ is an ...
... Other Elements on the periodic table • ____________ Transition Metals - elements in Group 3 through 12. These metals are hard and shiny, good conductors of electricity, less reactive than metals in Groups 1 and 2. ________ is an ...
Chapter 1 Matter on the Atomic Scale
... Nonmetals Occur in all physical states. solids: sulfur, phosphorus, carbon. liquid: bromine. gases: oxygen, helium, nitrogen. ...
... Nonmetals Occur in all physical states. solids: sulfur, phosphorus, carbon. liquid: bromine. gases: oxygen, helium, nitrogen. ...
Review Molecule: more than one atom, e.g., O2, H2, CO, H2O
... Chemical transformations involve forming new combinations of atoms in whole-number ratios, e.g., H2O = 2 H : 1 O. Atoms are conserved in chemical and physical transformations. 2 H2 ...
... Chemical transformations involve forming new combinations of atoms in whole-number ratios, e.g., H2O = 2 H : 1 O. Atoms are conserved in chemical and physical transformations. 2 H2 ...
Academic Chemistry
... D. 24 uE. 34 u 8. Two isotopes of an element have different ________ A. Atomic numbers B. numbers of electrons C. mass numbers D. numbers of protons ...
... D. 24 uE. 34 u 8. Two isotopes of an element have different ________ A. Atomic numbers B. numbers of electrons C. mass numbers D. numbers of protons ...
document
... “varying ratio”. This means that there is more than one way to combine the ingredients. • In addition, the ingredients are physically mixed but not chemically combined and they can be easily ...
... “varying ratio”. This means that there is more than one way to combine the ingredients. • In addition, the ingredients are physically mixed but not chemically combined and they can be easily ...
ch3 B - Manasquan Public Schools
... Most reactive metals one valence eFound as compounds (salts) and not elements due to reactivity. As elements they are soft metals and good conductors. ...
... Most reactive metals one valence eFound as compounds (salts) and not elements due to reactivity. As elements they are soft metals and good conductors. ...
ATOMS and PERIODIC TABLE - John Q. Adams Middle School
... compounds which have metal atoms bonded with nonmetal atoms Covalent bonds are when two or more atoms share electrons Usually present with two or more nonmetals ...
... compounds which have metal atoms bonded with nonmetal atoms Covalent bonds are when two or more atoms share electrons Usually present with two or more nonmetals ...
Notes. - Net Start Class
... charged mixture of ions and electrons. Particles that have been ionized (charged) There exist at extremely high temperatures. Exist at naturally (stars) or man-made (neon signs). While plasma is the most abundant phase of matter in the universe, on earth it only occurs in a few limited places. ...
... charged mixture of ions and electrons. Particles that have been ionized (charged) There exist at extremely high temperatures. Exist at naturally (stars) or man-made (neon signs). While plasma is the most abundant phase of matter in the universe, on earth it only occurs in a few limited places. ...
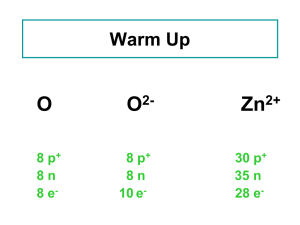
Atomic number
... Neutron: neutral particle in the nucleus Electron: tiny negative charge outside the nucleus Atoms are mostly…. … empty space! Element: a substance made of only one kind of atom, cannot be chemically or physically separated into other substances. ...
... Neutron: neutral particle in the nucleus Electron: tiny negative charge outside the nucleus Atoms are mostly…. … empty space! Element: a substance made of only one kind of atom, cannot be chemically or physically separated into other substances. ...
Element
... Atoms: the smallest component of an element which carries the chemical characteristics. Composed of a nucleus surrounded by electrons. An atom contains specific number of protons, neutrons, and electrons. ...
... Atoms: the smallest component of an element which carries the chemical characteristics. Composed of a nucleus surrounded by electrons. An atom contains specific number of protons, neutrons, and electrons. ...
The Nature of Matter
... • Balances out protons positive charge • In constant motion • Valence electrons are in outermost shell • Valence electrons determine the chemical nature of an atom • Smallest subatomic particle ...
... • Balances out protons positive charge • In constant motion • Valence electrons are in outermost shell • Valence electrons determine the chemical nature of an atom • Smallest subatomic particle ...
Chapter 4 Test Review
... • James Chadwick received the Nobel Prize in 1935 for discovering the existence of neutrons, neutral particles in the nucleus which accounts for the remainder of an atom’s mass. ...
... • James Chadwick received the Nobel Prize in 1935 for discovering the existence of neutrons, neutral particles in the nucleus which accounts for the remainder of an atom’s mass. ...
Unit 3C Standards for Quiz
... It will be similar to the last exam but there will be at least three questions per standard. Remember that since no calculators are allowed on the standards exam that we will be modeling this in this assessment of progress. Atomic and Molecular Structure 1. The Periodic Table displays the elements i ...
... It will be similar to the last exam but there will be at least three questions per standard. Remember that since no calculators are allowed on the standards exam that we will be modeling this in this assessment of progress. Atomic and Molecular Structure 1. The Periodic Table displays the elements i ...
Basic atomic structure
... + Charge Used to identify an atom Equal to atomic number Included in mass number Same mass as a neutron Change in protons [RARE!] = change in element name and atomic mass ...
... + Charge Used to identify an atom Equal to atomic number Included in mass number Same mass as a neutron Change in protons [RARE!] = change in element name and atomic mass ...
Lecture
... the binding energy is(not) great enough to hold the nucleus together. Unstable atoms will lose neutrons or protons as they attempt to become stable. They are called radioactive atoms. What is a radioactive decay? o spontaneous breakdown of an atomic nucleus resulting in the release of nuclear radiat ...
... the binding energy is(not) great enough to hold the nucleus together. Unstable atoms will lose neutrons or protons as they attempt to become stable. They are called radioactive atoms. What is a radioactive decay? o spontaneous breakdown of an atomic nucleus resulting in the release of nuclear radiat ...
Section 2 Powerpoint
... of the protons and neutrons in the nucleus of that atom. • An atom of aluminum with 13 protons and 14 neutrons has a mass number of 27. • If you know the atomic number and the mass number of an atom, you can find the number of neutrons by subtracting. Number of neutrons = Mass number – Atomic number ...
... of the protons and neutrons in the nucleus of that atom. • An atom of aluminum with 13 protons and 14 neutrons has a mass number of 27. • If you know the atomic number and the mass number of an atom, you can find the number of neutrons by subtracting. Number of neutrons = Mass number – Atomic number ...
Ch 2-1 Properties of Matter
... 71) A gas may be released during a physical change. For example, bubbles form when water boils. 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a ...
... 71) A gas may be released during a physical change. For example, bubbles form when water boils. 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a ...
Bohr´s atomic model (1913)
... Electrons orbit around the nucleus (which, as we know now, is formed by protons and neutrons) in different layers. In each layer there is a maximum number of electrons: In the first layer there are 2 electrons at most, 8 in the second layer, 18 in the third layer... In the layer n there are 2n2 elec ...
... Electrons orbit around the nucleus (which, as we know now, is formed by protons and neutrons) in different layers. In each layer there is a maximum number of electrons: In the first layer there are 2 electrons at most, 8 in the second layer, 18 in the third layer... In the layer n there are 2n2 elec ...
Chemistry Final - Practice Test I
... An atom of one or more substances are rearranged to form different substances b. Law of Definite Proportions (not Law of Conservation of Matter) A compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound. ...
... An atom of one or more substances are rearranged to form different substances b. Law of Definite Proportions (not Law of Conservation of Matter) A compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound. ...
electron
... mass 10.012 amu and a relative abundance of 19.91%. The isotope with mass 11.009 amu has a relative abundance of 80.09%. 1. Calculate the atomic mass of this element (show all work) and then name this element. ...
... mass 10.012 amu and a relative abundance of 19.91%. The isotope with mass 11.009 amu has a relative abundance of 80.09%. 1. Calculate the atomic mass of this element (show all work) and then name this element. ...
8th Grade
... _________________________________________________________________________________________ 16. In 1800 another scientist added to the idea of the Greek guy. Who was this man and what was his idea? _________________________________________________________________________________________ ______________ ...
... _________________________________________________________________________________________ 16. In 1800 another scientist added to the idea of the Greek guy. Who was this man and what was his idea? _________________________________________________________________________________________ ______________ ...
Atomic Mass - AJS Phyiscs and Chemistry
... • Dmitiri Mendeleev organized the elements in a way that reflected these common properties. • He wrote all the properties of each element known at the time on a card and laid them out on a table to find a pattern. • He came upon a layout that followed an increasing atomic mass and seemed to group e ...
... • Dmitiri Mendeleev organized the elements in a way that reflected these common properties. • He wrote all the properties of each element known at the time on a card and laid them out on a table to find a pattern. • He came upon a layout that followed an increasing atomic mass and seemed to group e ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.