Stoichiometry
... 1. Write the skeletal equation 2. Balance one element at a time using coefficients – Start with the elements in the most complex substance and finish with those in the least complex one – Alternatively, start with the element present in the fewest number of formulas and finish with the element prese ...
... 1. Write the skeletal equation 2. Balance one element at a time using coefficients – Start with the elements in the most complex substance and finish with those in the least complex one – Alternatively, start with the element present in the fewest number of formulas and finish with the element prese ...
Chemistry MCQS 12 class
... (CaSO4.2H2O, 2CaSO4.H2O, (CaSO4)2H2O) 18. The atoms of the elements belonging to the same period of the Periodic table have __________. (Same number of protons, same number of neutrons, same number of valence shells) 19. Sodium thiosulphate is used in photography because of its __________. ...
... (CaSO4.2H2O, 2CaSO4.H2O, (CaSO4)2H2O) 18. The atoms of the elements belonging to the same period of the Periodic table have __________. (Same number of protons, same number of neutrons, same number of valence shells) 19. Sodium thiosulphate is used in photography because of its __________. ...
Devillez (ld2653) – Test 1 Review – Devillez – (99998)
... 3. Two or more kinds of atoms may combine in different ways to form more than one kind of chemical compound. 4. Atoms of a given element all weigh the same. ...
... 3. Two or more kinds of atoms may combine in different ways to form more than one kind of chemical compound. 4. Atoms of a given element all weigh the same. ...
Chapter 3:Mass Relationships in Chemical Reactions
... H2 (g) + Cl2 (g) HCl (g) • Notice the subscript for H and Cl is 2, therefore we have 2 atoms of each substance. In the products, we have HCl, 1 atom of each. We can balance the equation by putting a 2 in front HCl. H2 (g) + Cl2(g) 2 HCl (g) ...
... H2 (g) + Cl2 (g) HCl (g) • Notice the subscript for H and Cl is 2, therefore we have 2 atoms of each substance. In the products, we have HCl, 1 atom of each. We can balance the equation by putting a 2 in front HCl. H2 (g) + Cl2(g) 2 HCl (g) ...
CH_3 IN TOTO - WordPress.com
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
PS 2 - Purdyphysicalscience
... Design a poster illustrating ONE of the models that were proposed since 1800s ...
... Design a poster illustrating ONE of the models that were proposed since 1800s ...
Chapter 3
... NOT in weight ratios !! When in doubt, convert to moles !!! CH4 + 2 O2 CO2 + 2 H2O Get a set of relationships between all reactants and products: 1 mol CH4 = 2 mol O2 = 1 mol CO2 = 2 mol H2O ...
... NOT in weight ratios !! When in doubt, convert to moles !!! CH4 + 2 O2 CO2 + 2 H2O Get a set of relationships between all reactants and products: 1 mol CH4 = 2 mol O2 = 1 mol CO2 = 2 mol H2O ...
Matter Flashcards 5 - Henrico County Public Schools
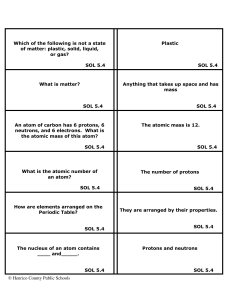
... A molecule of an element is two or more atoms of the same kind of element joined together. A molecule of a compound is made of different kinds of elements joined together. SOL 5.4 A mixture is two or more substances joined physically. A compound is two or more substances joined ...
... A molecule of an element is two or more atoms of the same kind of element joined together. A molecule of a compound is made of different kinds of elements joined together. SOL 5.4 A mixture is two or more substances joined physically. A compound is two or more substances joined ...
Major 01 - KFUPM Faculty List
... In the exams because of an error in the program there were 2 choices identical as N2O5. However, since N2O5 is wrong anyway, this is no problem. Avogadro's law says, that at the same T and P equal volumes of gases contain equal numbers of molecules. Thus, assuming that 1 L N2 contains n molecules N ...
... In the exams because of an error in the program there were 2 choices identical as N2O5. However, since N2O5 is wrong anyway, this is no problem. Avogadro's law says, that at the same T and P equal volumes of gases contain equal numbers of molecules. Thus, assuming that 1 L N2 contains n molecules N ...
A Dictionary of the New Chymical Nomenclature
... Sulphureous acid Volatile sulphureous acid Phlogisticated vitriolic acid Spirit of sulphur ...
... Sulphureous acid Volatile sulphureous acid Phlogisticated vitriolic acid Spirit of sulphur ...
File
... and P. Carbon also forms bonds. Silicon, on the other hand, forms a very strong single bond with oxygen and does not form bonds. Silicon would rather have SiO bonds than any other type of bond, including multiple bonds. This is unlike carbon. The two major allotropic forms of carbon are graphit ...
... and P. Carbon also forms bonds. Silicon, on the other hand, forms a very strong single bond with oxygen and does not form bonds. Silicon would rather have SiO bonds than any other type of bond, including multiple bonds. This is unlike carbon. The two major allotropic forms of carbon are graphit ...
Chapter 5: Calculations and the Chemical Equation
... determining the composition. For example, dihydrogen monoxide (water, H2O) is a compound composed of two hydrogen atoms for every oxygen atom. The Chemical Formula A chemical formula (also called molecular formula) is a concise way of expressing information about the atoms that constitute a particul ...
... determining the composition. For example, dihydrogen monoxide (water, H2O) is a compound composed of two hydrogen atoms for every oxygen atom. The Chemical Formula A chemical formula (also called molecular formula) is a concise way of expressing information about the atoms that constitute a particul ...
Into the Atom - Structure of the Nucleus, Heavy Nuclei... Original Script and research: Dr. Arvind Dubey
... in stars through the process of nuclear fusion to produce more of the element helium, and the sequence of elements from carbon up to iron. Almost all the atoms present on Earth today were present in the nebula from which your solar system was created. Some of my present companions were not around at ...
... in stars through the process of nuclear fusion to produce more of the element helium, and the sequence of elements from carbon up to iron. Almost all the atoms present on Earth today were present in the nebula from which your solar system was created. Some of my present companions were not around at ...
DOE Chemistry 1
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
Chemistry: Percent Yield
... proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be calculated mathematically 37: 3.3iv ...
... proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be calculated mathematically 37: 3.3iv ...
4.1 Defining the Atom
... simple ideas about matter and electric charges. 1. Atoms have no net electric charge; they are electrically neutral. 2. Electric charges are carried by particles of matter. 3. Electric charges always exist in whole-number multiples of a single basic unit; that is, there are no fractions of charges. ...
... simple ideas about matter and electric charges. 1. Atoms have no net electric charge; they are electrically neutral. 2. Electric charges are carried by particles of matter. 3. Electric charges always exist in whole-number multiples of a single basic unit; that is, there are no fractions of charges. ...
FREE Sample Here
... 1) According to history, the concept that all matter is composed of atoms was first proposed by A) the Greek philosopher Democritus, but not widely accepted until modern times. B) Dalton, but not widely accepted until the work of Mendeleev. C) Dalton, but not widely accepted until the work of Einste ...
... 1) According to history, the concept that all matter is composed of atoms was first proposed by A) the Greek philosopher Democritus, but not widely accepted until modern times. B) Dalton, but not widely accepted until the work of Mendeleev. C) Dalton, but not widely accepted until the work of Einste ...
FREE Sample Here
... 1) According to history, the concept that all matter is composed of atoms was first proposed by A) the Greek philosopher Democritus, but not widely accepted until modern times. B) Dalton, but not widely accepted until the work of Mendeleev. C) Dalton, but not widely accepted until the work of Einste ...
... 1) According to history, the concept that all matter is composed of atoms was first proposed by A) the Greek philosopher Democritus, but not widely accepted until modern times. B) Dalton, but not widely accepted until the work of Mendeleev. C) Dalton, but not widely accepted until the work of Einste ...
chemistry-with-masteringchemistry-6th-edition-mcmurry-test-bank
... 1) According to history, the concept that all matter is composed of atoms was first proposed by A) the Greek philosopher Democritus, but not widely accepted until modern times. B) Dalton, but not widely accepted until the work of Mendeleev. C) Dalton, but not widely accepted until the work of Einste ...
... 1) According to history, the concept that all matter is composed of atoms was first proposed by A) the Greek philosopher Democritus, but not widely accepted until modern times. B) Dalton, but not widely accepted until the work of Mendeleev. C) Dalton, but not widely accepted until the work of Einste ...
ch03 - Atoms and Elements
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
Organic Chemistry Curriculum Map - Belle Vernon Area School District
... CHEM.A.2.1.2 – Differentiate between the mass number of an isotope and the average atomic mass of an element. Anchor: CHEM.A.2.3 – Explain how periodic trends in the properties of atoms allow for the prediction of physical and chemical properties. Eligible Content CHEM.A.2.3.2 – Explain how the ...
... CHEM.A.2.1.2 – Differentiate between the mass number of an isotope and the average atomic mass of an element. Anchor: CHEM.A.2.3 – Explain how periodic trends in the properties of atoms allow for the prediction of physical and chemical properties. Eligible Content CHEM.A.2.3.2 – Explain how the ...
File
... 37. The hydrides formed by the transfer of electrons from electropositive metals to hydrogen are called __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Interstitial hydrides) 38. NaH is an example of __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Interstitial hyd ...
... 37. The hydrides formed by the transfer of electrons from electropositive metals to hydrogen are called __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Interstitial hydrides) 38. NaH is an example of __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Interstitial hyd ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.