Basics of Chemistry
... home economics, engineering, geology, physics, biology, medicine, and dentistry. In this section we shall describe the simplest atomic theory. We shall use it as we represent chemical formulas of elements and compounds. Later this theory will be expanded when we discuss chemical changes. The word “s ...
... home economics, engineering, geology, physics, biology, medicine, and dentistry. In this section we shall describe the simplest atomic theory. We shall use it as we represent chemical formulas of elements and compounds. Later this theory will be expanded when we discuss chemical changes. The word “s ...
View PDF
... The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. ...
... The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. ...
File
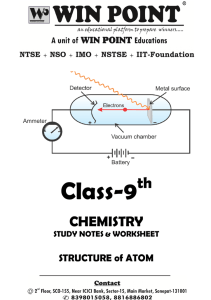
... comb gets an electric charge, and on rubbing with silk cloth, a glass rod also gets an electric charge. 1.1 Discovery Of Electron In 1830, Michael Faraday showed that if electricity is passed through a solution of an electrolyte, chemical reactions occurred at the electrodes, which resulted in the l ...
... comb gets an electric charge, and on rubbing with silk cloth, a glass rod also gets an electric charge. 1.1 Discovery Of Electron In 1830, Michael Faraday showed that if electricity is passed through a solution of an electrolyte, chemical reactions occurred at the electrodes, which resulted in the l ...
Chemistry - Sanskriti School
... classification and IUPAC . Nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyper conjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions; electrophiles and nucleoph ...
... classification and IUPAC . Nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyper conjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions; electrophiles and nucleoph ...
Introduction to Atomic Structure - New Jersey Center for Teaching
... This material is made freely available at www.njctl.org and is intended for the non-commercial use of students and teachers. These materials may not be used for any commercial purpose without the written permission of the owners. NJCTL maintains its website for the convenience of teachers who wish t ...
... This material is made freely available at www.njctl.org and is intended for the non-commercial use of students and teachers. These materials may not be used for any commercial purpose without the written permission of the owners. NJCTL maintains its website for the convenience of teachers who wish t ...
Chemistry 3.3 teacher led revised
... The mass of the reactants is the same as the total mass of the products. Matter cannot be created or destroyed. 2. How does NaCl (table salt) follow the Law of Definite Proportions? It is always made up of same ratio of element which is 39.34% Na and 60.66% Cl. 3. How does the diagram depict the Law ...
... The mass of the reactants is the same as the total mass of the products. Matter cannot be created or destroyed. 2. How does NaCl (table salt) follow the Law of Definite Proportions? It is always made up of same ratio of element which is 39.34% Na and 60.66% Cl. 3. How does the diagram depict the Law ...
Section 2.7 An Introduction to the Periodic Table
... is within reach of chemical agency. We might as well try to introduce a new planet into the solar system and to annihilate one already in existence, as to create or destroy a particle of hydrogen- J.D. Return to TOC ...
... is within reach of chemical agency. We might as well try to introduce a new planet into the solar system and to annihilate one already in existence, as to create or destroy a particle of hydrogen- J.D. Return to TOC ...
FREE Sample Here
... deflected. Realizing that atoms are electrically neutral (that is, they have equal numbers of protons and electrons) and that the mass of a proton is significantly greater than the mass of an electron, use Rutherford's data to propose a structural model of an atom. ...
... deflected. Realizing that atoms are electrically neutral (that is, they have equal numbers of protons and electrons) and that the mass of a proton is significantly greater than the mass of an electron, use Rutherford's data to propose a structural model of an atom. ...
Shedding Light on Atoms Episode 5: Protons, Neutrons, and Electrons
... and neutrons, or nucleons, in an atom is called the atom’s mass number, and, somewhat confusingly in English, is given the symbol A. All these different forms of the same type of atom are called isotopes. Here we’re showing three isotopes of carbon. They all chemically react the same way, because th ...
... and neutrons, or nucleons, in an atom is called the atom’s mass number, and, somewhat confusingly in English, is given the symbol A. All these different forms of the same type of atom are called isotopes. Here we’re showing three isotopes of carbon. They all chemically react the same way, because th ...
Chapter 3 Stoichiometry: Calculations with Chemical
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
Atoms: The Building Blocks of Matter - Milton
... Section 1 covers the history and development of atomic theory, from Democritus to Dalton to the modern era. Section 2 covers the experiments that led to the discovery of the electron and the nucleus as well as the principal properties of these subatomic particles. ...
... Section 1 covers the history and development of atomic theory, from Democritus to Dalton to the modern era. Section 2 covers the experiments that led to the discovery of the electron and the nucleus as well as the principal properties of these subatomic particles. ...
Chem 110 2014 - University of KwaZulu
... Isotopes - atoms of same element having d different numbers of nO in their nuclei ie. same Z, different A, or same Z, different N - chemical properties largely similar, but physical properties, & particularly the ones involving radioactive “nuclei”, can be very different - each Mg atom is one of th ...
... Isotopes - atoms of same element having d different numbers of nO in their nuclei ie. same Z, different A, or same Z, different N - chemical properties largely similar, but physical properties, & particularly the ones involving radioactive “nuclei”, can be very different - each Mg atom is one of th ...
Chemistry Standards Clarification
... Describe the distinctions between scientific theories, laws, hypotheses, and observations. Explain the progression of ideas and explanations that lead to science theories that are part of the current scientific consensus or core knowledge. Apply science principles or scientific data to anticipate ef ...
... Describe the distinctions between scientific theories, laws, hypotheses, and observations. Explain the progression of ideas and explanations that lead to science theories that are part of the current scientific consensus or core knowledge. Apply science principles or scientific data to anticipate ef ...
Atomic Polar Tensor Transferabllity and Atomic Charges kr the
... in ref 1. (RtY)represents the center of charge of the h brid orbital (pv),where p and v indicate orbitals of atom A, and R,,YB represents the bonding center of charge since p and v belong to different atoms, A and B, whether chemically bonded or not. These contributions in expression 1 are known, re ...
... in ref 1. (RtY)represents the center of charge of the h brid orbital (pv),where p and v indicate orbitals of atom A, and R,,YB represents the bonding center of charge since p and v belong to different atoms, A and B, whether chemically bonded or not. These contributions in expression 1 are known, re ...
M for Moles - Shop
... List of Relative Atomic Mass How to use this e-book Finding calculations in chemistry difficult to learn? Don’t know how/when to or why use moles in chemistry? Confuse about molecular weights and their relationships to moles and chemical equations? This e-book teaches you how to do mole calculations ...
... List of Relative Atomic Mass How to use this e-book Finding calculations in chemistry difficult to learn? Don’t know how/when to or why use moles in chemistry? Confuse about molecular weights and their relationships to moles and chemical equations? This e-book teaches you how to do mole calculations ...
chap-4-atomic-weights
... particles (H2, O2, CH4, C2H6, C2H2, C3H8, etc) were the same size. But this sounds, ah, stupid. How could a particle of CH4 be the same size as a particle of C3H8? But it wasn’t really stupid - because Avogadro’s idea was that most of a gas might really be empty space! (He thought this because gases ...
... particles (H2, O2, CH4, C2H6, C2H2, C3H8, etc) were the same size. But this sounds, ah, stupid. How could a particle of CH4 be the same size as a particle of C3H8? But it wasn’t really stupid - because Avogadro’s idea was that most of a gas might really be empty space! (He thought this because gases ...
History of the Atom
... –Actually proposed the word atom (indivisible) because he believed that all matter consisted of such tiny units with voids between, an idea quite similar to our own beliefs. It was rejected by Aristotle and thus lost for 2000 years. ...
... –Actually proposed the word atom (indivisible) because he believed that all matter consisted of such tiny units with voids between, an idea quite similar to our own beliefs. It was rejected by Aristotle and thus lost for 2000 years. ...
chapter - Grygla School
... Rutherford Discovered the Nucleus Thomson proposed that the electrons of an atom were embedded in a positively charged ball of matter. His picture of an atom, which is shown in Figure 7, was named the plum-pudding model because it resembled plum pudding, a dessert consisting of a ball of cake with ...
... Rutherford Discovered the Nucleus Thomson proposed that the electrons of an atom were embedded in a positively charged ball of matter. His picture of an atom, which is shown in Figure 7, was named the plum-pudding model because it resembled plum pudding, a dessert consisting of a ball of cake with ...
Chapter 2 - Atoms and the Periodic Table (test bank)
... A. Rutherford discovered the atomic nucleus by bombarding gold foil with electrons B. The proton and the neutron have identical masses. C. The neutron's mass is equal to that of a proton plus an electron. D. A neutral atom contains equal numbers of protons and electrons. E. An atomic nucleus contain ...
... A. Rutherford discovered the atomic nucleus by bombarding gold foil with electrons B. The proton and the neutron have identical masses. C. The neutron's mass is equal to that of a proton plus an electron. D. A neutral atom contains equal numbers of protons and electrons. E. An atomic nucleus contain ...
Chapter 2 Atoms, Molecules, and Ions
... Given the above data, what is the average molecular mass of magnesium (Mg)? ...
... Given the above data, what is the average molecular mass of magnesium (Mg)? ...
Chapter 1
... liquid must be changed into a gas. Why is boiling point considered a physical property when a gas’ appearance is much different from that of a liquid? Although the appearance is different, the substance is still the same. Its chemical identity remains the same irrespective of the physical state. Ret ...
... liquid must be changed into a gas. Why is boiling point considered a physical property when a gas’ appearance is much different from that of a liquid? Although the appearance is different, the substance is still the same. Its chemical identity remains the same irrespective of the physical state. Ret ...
Introduction to chemistry Multiple Choice 1. Which SI prefix means
... 96. The mass of a substance is independent of its location. Answer: True; Difficulty: easy; Reference: Section 2.7 97. The density of liquid A is 2.14g/mL and the density of liquid B is 1.46g/mL. When equal masses of these liquids are compared, liquid A will have the greater volume. Answer: False; D ...
... 96. The mass of a substance is independent of its location. Answer: True; Difficulty: easy; Reference: Section 2.7 97. The density of liquid A is 2.14g/mL and the density of liquid B is 1.46g/mL. When equal masses of these liquids are compared, liquid A will have the greater volume. Answer: False; D ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.