* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download History of the Atom
Survey
Document related concepts
Transcript
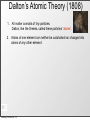
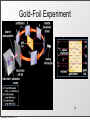
History of the Atom Scientists and Their Contribution to the Model of an Atom Tuesday, October 8, 13 History of the Atom - Timeline 0 Tuesday, October 8, 13 0 Tuesday, October 8, 13 1700s 1800s 1900s History of the Atom - Timeline History of the Atom - Timeline 0 Click on picture for more information Tuesday, October 8, 13 1700s 1800s 1900s 460 – 370 BC History of the Atom - Timeline 0 Democritus proposes the 1st atomic theory Click on picture for more information Tuesday, October 8, 13 1700s 1800s 1900s 460 – 370 BC History of the Atom - Timeline 0 Click on picture for more information Tuesday, October 8, 13 1700s 1800s 1900s 460 – 370 BC History of the Atom - Timeline 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 History of the Atom - Timeline Antoine Lavoisier makes a substantial number of contributions to the field of Chemistry 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 History of the Atom - Timeline 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 History of the Atom - Timeline 1766 – 1844 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 History of the Atom - Timeline 1766 – 1844 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 John Dalton proposes his atomic theory in 1803 History of the Atom - Timeline 1766 – 1844 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 History of the Atom - Timeline 1766 – 1844 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 J.J. Thomson discovers the electron and proposes the Plum Pudding Model in 1897 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1700s 1800s 1900s 460 – 370 BC 0 Ernest Rutherford performs the Gold Foil Experiment in 1909 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 Niels Bohr proposes the Bohr Model in 1913 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1887 – 1961 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1887 – 1961 1700s 1800s 1900s 460 – 370 BC 0 Erwin Schrodinger describes the electron cloud in 1926 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1887 – 1961 1700s 1800s 1900s 460 – 370 BC 0 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1887 – 1961 1700s 1800s 1900s 460 – 370 BC 0 1891 – 1974 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1887 – 1961 1700s 1800s 1900s 460 – 370 BC James Chadwick discovered the neutron in in 1932 0 1891 – 1974 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 History of the Atom - Timeline 1766 – 1844 1871 – 1937 1887 – 1961 1700s 1800s 1900s 460 – 370 BC 0 1891 – 1974 1743 – 1794 1885 – 1962 Click on picture for more information Tuesday, October 8, 13 1856 – 1940 Democritus (460 BC – 370 BC) Tuesday, October 8, 13 Democritus (460 BC – 370 BC) Image taken from: https://reichchemistry.wikispaces.com/T.+Glenn +Time+Line+Project Tuesday, October 8, 13 Democritus (460 BC – 370 BC) • Proposed an Atomic Theory (along with his mentor Leucippus) which states that all atoms are small, hard, indivisible and indestructible particles made of a single material formed into different shapes and sizes. Tuesday, October 8, 13 Image taken from: https://reichchemistry.wikispaces.com/T.+Glenn +Time+Line+Project Democritus (460 BC – 370 BC) • Proposed an Atomic Theory (along with his mentor Leucippus) which states that all atoms are small, hard, indivisible and indestructible particles made of a single material formed into different shapes and sizes. • Aristotle did not support his atomic theory Tuesday, October 8, 13 Image taken from: https://reichchemistry.wikispaces.com/T.+Glenn +Time+Line+Project Some Early Ideas on Matter Tuesday, October 8, 13 Some Early Ideas on Matter Anaxagoras (Greek, born 500 B.C.) –Suggested every substance had its own kind of “seeds” that clustered together to make the substance, much as our atoms cluster to make molecules. Tuesday, October 8, 13 Some Early Ideas on Matter Anaxagoras (Greek, born 500 B.C.) –Suggested every substance had its own kind of “seeds” that clustered together to make the substance, much as our atoms cluster to make molecules. Empedocles (Greek, born in Sicily, 490 B.C.) –Suggested there were only four basic seeds – earth, air, fire, and water. The elementary substances (atoms to us) combined in various ways to make everything. Tuesday, October 8, 13 Some Early Ideas on Matter Anaxagoras (Greek, born 500 B.C.) –Suggested every substance had its own kind of “seeds” that clustered together to make the substance, much as our atoms cluster to make molecules. Empedocles (Greek, born in Sicily, 490 B.C.) –Suggested there were only four basic seeds – earth, air, fire, and water. The elementary substances (atoms to us) combined in various ways to make everything. Democritus (Greek, born 470 B.C.) –Actually proposed the word atom (indivisible) because he believed that all matter consisted of such tiny units with voids between, an idea quite similar to our own beliefs. It was rejected by Aristotle and thus lost for 2000 years. Tuesday, October 8, 13 Some Early Ideas on Matter Anaxagoras (Greek, born 500 B.C.) –Suggested every substance had its own kind of “seeds” that clustered together to make the substance, much as our atoms cluster to make molecules. Empedocles (Greek, born in Sicily, 490 B.C.) –Suggested there were only four basic seeds – earth, air, fire, and water. The elementary substances (atoms to us) combined in various ways to make everything. Democritus (Greek, born 470 B.C.) –Actually proposed the word atom (indivisible) because he believed that all matter consisted of such tiny units with voids between, an idea quite similar to our own beliefs. It was rejected by Aristotle and thus lost for 2000 years. Aristotle (Greek, born 384 B.C.) –Added the idea of “qualities” – heat, cold, dryness, moisture – as basic elements which combined as shown in the diagram (previous page). Hot + dry made fire; hot + wet made air, and so on. Tuesday, October 8, 13 Some Early Ideas on Matter Anaxagoras (Greek, born 500 B.C.) –Suggested every substance had its own kind of “seeds” that clustered together to make the substance, much as our atoms cluster to make molecules. Empedocles (Greek, born in Sicily, 490 B.C.) –Suggested there were only four basic seeds – earth, air, fire, and water. The elementary substances (atoms to us) combined in various ways to make everything. Democritus (Greek, born 470 B.C.) –Actually proposed the word atom (indivisible) because he believed that all matter consisted of such tiny units with voids between, an idea quite similar to our own beliefs. It was rejected by Aristotle and thus lost for 2000 years. Aristotle (Greek, born 384 B.C.) –Added the idea of “qualities” – heat, cold, dryness, moisture – as basic elements which combined as shown in the diagram (previous page). Hot + dry made fire; hot + wet made air, and so on. Tuesday, October 8, 13 Alchemy Tuesday, October 8, 13 Alchemy • After that chemistry was ruled by alchemy. • They believed that they could take any cheap metals and turn them into gold. This is called transmutation. • Alchemists were almost like magicians. – elixirs, physical immortality Tuesday, October 8, 13 Contributions of alchemists: Tuesday, October 8, 13 Contributions of alchemists: Information about elements Tuesday, October 8, 13 Contributions of alchemists: Information about elements - the elements mercury, sulfur, and antimony were discovered Tuesday, October 8, 13 Contributions of alchemists: Information about elements - the elements mercury, sulfur, and antimony were discovered - properties of some elements Tuesday, October 8, 13 Contributions of alchemists: Information about elements - the elements mercury, sulfur, and antimony were discovered - properties of some elements Develop lab apparatus / procedures / experimental techniques Tuesday, October 8, 13 Contributions of alchemists: Information about elements - the elements mercury, sulfur, and antimony were discovered - properties of some elements Develop lab apparatus / procedures / experimental techniques - alchemists learned how to prepare acids. Tuesday, October 8, 13 Contributions of alchemists: Information about elements - the elements mercury, sulfur, and antimony were discovered - properties of some elements Develop lab apparatus / procedures / experimental techniques - alchemists learned how to prepare acids. - developed several alloys Tuesday, October 8, 13 Contributions of alchemists: Information about elements - the elements mercury, sulfur, and antimony were discovered - properties of some elements Develop lab apparatus / procedures / experimental techniques - alchemists learned how to prepare acids. - developed several alloys - new glassware Tuesday, October 8, 13 Antoine Lavoisier (1743 – 1794) Tuesday, October 8, 13 Antoine Lavoisier (1743 – 1794) Image taken from: www.ldeo.columbia.edu/.../v1001/ geotime2.html Tuesday, October 8, 13 Antoine Lavoisier (1743 – 1794) • Known as the “Father of Modern Chemistry” Image taken from: www.ldeo.columbia.edu/.../v1001/ geotime2.html Tuesday, October 8, 13 Antoine Lavoisier (1743 – 1794) • Known as the “Father of Modern Chemistry” • Was the first person to generate a list of thirty-three elements in his textbook Image taken from: www.ldeo.columbia.edu/.../v1001/ geotime2.html Tuesday, October 8, 13 Antoine Lavoisier (1743 – 1794) • Known as the “Father of Modern Chemistry” • Was the first person to generate a list of thirty-three elements in his textbook • Devised the metric system Image taken from: www.ldeo.columbia.edu/.../v1001/ geotime2.html Tuesday, October 8, 13 Antoine Lavoisier (1743 – 1794) • Known as the “Father of Modern Chemistry” • Was the first person to generate a list of thirty-three elements in his textbook • Devised the metric system • Was married to a 13-year old Marie-Anne Pierette Paulze; she assisted him with much of his work Image taken from: www.ldeo.columbia.edu/.../v1001/ geotime2.html Tuesday, October 8, 13 Antoine Lavoisier (1743 – 1794) • Known as the “Father of Modern Chemistry” • Was the first person to generate a list of thirty-three elements in his textbook • Devised the metric system • Was married to a 13-year old Marie-Anne Pierette Paulze; she assisted him with much of his work • Was a tax-collector that was consequently guillotined during the French Revolution Image taken from: www.ldeo.columbia.edu/.../v1001/ geotime2.html Tuesday, October 8, 13 Antoine Lavoisier (1743 – 1794) • Known as the “Father of Modern Chemistry” • Was the first person to generate a list of thirty-three elements in his textbook • Devised the metric system Image taken from: www.ldeo.columbia.edu/.../v1001/ geotime2.html Tuesday, October 8, 13 • Was married to a 13-year old Marie-Anne Pierette Paulze; she assisted him with much of his work • Was a tax-collector that was consequently guillotined during the French Revolution • Discovered/proposed that combustion occurs when oxygen combines with other elements Antoine Lavoisier (1743 – 1794) • Known as the “Father of Modern Chemistry” • Was the first person to generate a list of thirty-three elements in his textbook • Devised the metric system Image taken from: www.ldeo.columbia.edu/.../v1001/ geotime2.html Tuesday, October 8, 13 • Was married to a 13-year old Marie-Anne Pierette Paulze; she assisted him with much of his work • Was a tax-collector that was consequently guillotined during the French Revolution • Discovered/proposed that combustion occurs when oxygen combines with other elements • Discovered/proposed the Law of Conservation of Mass (or Matter) which states, in a chemical reaction, matter is neither created nor destroyed John Dalton (1766 – 1844) Tuesday, October 8, 13 John Dalton (1766 – 1844) Image taken from: chemistry.about.com/.../JohnDalton.htm Tuesday, October 8, 13 John Dalton (1766 – 1844) • In 1803, proposed an Atomic Theory which states: Image taken from: chemistry.about.com/.../JohnDalton.htm Tuesday, October 8, 13 John Dalton (1766 – 1844) • In 1803, proposed an Atomic Theory which states: o All substances are made of atoms; atoms are small particles that cannot be created, divided, or destroyed. Image taken from: chemistry.about.com/.../JohnDalton.htm Tuesday, October 8, 13 John Dalton (1766 – 1844) • In 1803, proposed an Atomic Theory which states: o All substances are made of atoms; atoms are small particles that cannot be created, divided, or destroyed. o Atoms of the same element are exactly alike, and atoms of different elements are different Image taken from: chemistry.about.com/.../JohnDalton.htm Tuesday, October 8, 13 John Dalton (1766 – 1844) • In 1803, proposed an Atomic Theory which states: o All substances are made of atoms; atoms are small particles that cannot be created, divided, or destroyed. o Atoms of the same element are exactly alike, and atoms of different elements are different o Atoms join with other atoms to make new substances Image taken from: chemistry.about.com/.../JohnDalton.htm Tuesday, October 8, 13 John Dalton (1766 – 1844) • In 1803, proposed an Atomic Theory which states: o All substances are made of atoms; atoms are small particles that cannot be created, divided, or destroyed. o Atoms of the same element are exactly alike, and atoms of different elements are different o Atoms join with other atoms to make new substances • Calculated the atomic weights of many various elements Tuesday, October 8, 13 Image taken from: chemistry.about.com/.../JohnDalton.htm John Dalton (1766 – 1844) • In 1803, proposed an Atomic Theory which states: o All substances are made of atoms; atoms are small particles that cannot be created, divided, or destroyed. o Atoms of the same element are exactly alike, and atoms of different elements are different o Atoms join with other atoms to make new substances • Calculated the atomic weights of many various elements • Was a teacher at a very young age Tuesday, October 8, 13 Image taken from: chemistry.about.com/.../JohnDalton.htm John Dalton (1766 – 1844) • In 1803, proposed an Atomic Theory which states: o All substances are made of atoms; atoms are small particles that cannot be created, divided, or destroyed. o Atoms of the same element are exactly alike, and atoms of different elements are different o Atoms join with other atoms to make new substances • Calculated the atomic weights of many various elements • Was a teacher at a very young age • Was color blind Tuesday, October 8, 13 Image taken from: chemistry.about.com/.../JohnDalton.htm Dalton’s Atomic Theory (1808) Tuesday, October 8, 13 Dalton’s Atomic Theory (1808) 1. All matter consists of tiny particles. Dalton, like the Greeks, called these particles “atoms”. Tuesday, October 8, 13 Dalton’s Atomic Theory (1808) 1. All matter consists of tiny particles. Dalton, like the Greeks, called these particles “atoms”. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. Tuesday, October 8, 13 Dalton’s Atomic Theory (1808) 1. All matter consists of tiny particles. Dalton, like the Greeks, called these particles “atoms”. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. 3. Atoms can neither be created nor destroyed. Tuesday, October 8, 13 Dalton’s Atomic Theory (1808) 1. All matter consists of tiny particles. Dalton, like the Greeks, called these particles “atoms”. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. 3. Atoms can neither be created nor destroyed. 4. All atoms of the same element are identical in mass, size, and other properties. Tuesday, October 8, 13 Dalton’s Atomic Theory (1808) 1. All matter consists of tiny particles. Dalton, like the Greeks, called these particles “atoms”. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. 3. Atoms can neither be created nor destroyed. 4. All atoms of the same element are identical in mass, size, and other properties. 5. Atoms of one element differ in mass and other properties from atoms of other elements. Tuesday, October 8, 13 Dalton’s Atomic Theory (1808) 1. All matter consists of tiny particles. Dalton, like the Greeks, called these particles “atoms”. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. 3. Atoms can neither be created nor destroyed. 4. All atoms of the same element are identical in mass, size, and other properties. 5. Atoms of one element differ in mass and other properties from atoms of other elements. 6. In compounds, atoms of different elements combine in simple, whole number ratios. Tuesday, October 8, 13 Dalton’s Atomic Theory (1808) 1. All matter consists of tiny particles. Dalton, like the Greeks, called these particles “atoms”. 2. Atoms of one element can neither be subdivided nor changed into atoms of any other element. 3. Atoms can neither be created nor destroyed. 4. All atoms of the same element are identical in mass, size, and other properties. 5. Atoms of one element differ in mass and other properties from atoms of other elements. 6. In compounds, atoms of different elements combine in simple, whole number ratios. Tuesday, October 8, 13 10 Tuesday, October 8, 13 Dalton’s symbols, 1808 Tuesday, October 8, 13 Dalton’s atomic weights, 1808 Tuesday, October 8, 13 Michael Faraday (1791-1867) • Michael Faraday (1825)- Split compounds by electrolysis and showed that atoms have electrical properties • 1833 Michael Faraday discovers quantitative laws of electrochemical deposition. • He introduces the terms electrode, cathode, anode, ion, anion, cation, electrolyte. • He establishes that a definite quantity13 of electricity is associated with each atom of Tuesday, October 8, 13 J.J. Thomson (1856 – 1940) Tuesday, October 8, 13 J.J. Thomson (1856 – 1940) Image taken from: www.wired.com/.../news/2008/04/ dayintech_0430 Tuesday, October 8, 13 J.J. Thomson (1856 – 1940) • Proved that an atom can be divided into smaller parts Image taken from: www.wired.com/.../news/2008/04/ dayintech_0430 Tuesday, October 8, 13 J.J. Thomson (1856 – 1940) • Proved that an atom can be divided into smaller parts • While experimenting with cathoderay tubes, discovered corpuscles, which were later called electrons Image taken from: www.wired.com/.../news/2008/04/ dayintech_0430 Tuesday, October 8, 13 J.J. Thomson (1856 – 1940) • Proved that an atom can be divided into smaller parts • While experimenting with cathoderay tubes, discovered corpuscles, which were later called electrons • Stated that the atom is neutral Image taken from: www.wired.com/.../news/2008/04/ dayintech_0430 Tuesday, October 8, 13 J.J. Thomson (1856 – 1940) • Proved that an atom can be divided into smaller parts • While experimenting with cathoderay tubes, discovered corpuscles, which were later called electrons • Stated that the atom is neutral Image taken from: www.wired.com/.../news/2008/04/ dayintech_0430 Tuesday, October 8, 13 • In 1897, proposed the Plum Pudding Model which states that atoms mostly consist of positively charged material with negatively charged particles (electrons) located throughout the positive material J.J. Thomson (1856 – 1940) • Proved that an atom can be divided into smaller parts • While experimenting with cathoderay tubes, discovered corpuscles, which were later called electrons • Stated that the atom is neutral Image taken from: www.wired.com/.../news/2008/04/ dayintech_0430 Tuesday, October 8, 13 • In 1897, proposed the Plum Pudding Model which states that atoms mostly consist of positively charged material with negatively charged particles (electrons) located throughout the positive material • Won a Nobel Prize Discovered by J.J. Thomson. He said the atom was a sphere of positive electricity, with negative particles throughout. This came around right after he discovered the electron. Tuesday, October 8, 13 Robert Millikan http://www.chemistryexplained.com/images/chfa_03_img0536.jpg He was by far the most famous American scientist. He wanted to find the electrical charge of electrons. He measured water droplets, and that wasn’t successful, so he measured oil droplets, where all this proved electrons were negatively charged. He also was a professor for many years, and wrote many textbooks on chemistry. Tuesday, October 8, 13 • Robert Millikan (1868-1923) – Oil drop experiment – 1909 Tuesday, October 8, 13 Ernest Rutherford (1871 – 1937) Tuesday, October 8, 13 Ernest Rutherford (1871 – 1937) Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Tuesday, October 8, 13 Ernest Rutherford (1871 – 1937) • In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Tuesday, October 8, 13 Ernest Rutherford (1871 – 1937) • In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: o It consists of a small core, or nucleus, that contains most of the mass of the atom Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Tuesday, October 8, 13 Ernest Rutherford (1871 – 1937) • In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: o It consists of a small core, or nucleus, that contains most of the mass of the atom o This nucleus is made up of particles called protons, which have a positive charge Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Tuesday, October 8, 13 Ernest Rutherford (1871 – 1937) • In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: o It consists of a small core, or nucleus, that contains most of the mass of the atom o This nucleus is made up of particles called protons, which have a positive charge o The protons are surrounded by negatively charged electrons, but most of the atom is actually empty space Tuesday, October 8, 13 Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Ernest Rutherford (1871 – 1937) • • In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: o It consists of a small core, or nucleus, that contains most of the mass of the atom o This nucleus is made up of particles called protons, which have a positive charge o The protons are surrounded by negatively charged electrons, but most of the atom is actually empty space Did extensive work on radioactivity (alpha & beta particles, gamma rays/waves) and was referred to as the “Father of Nuclear Physics” Tuesday, October 8, 13 Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Ernest Rutherford (1871 – 1937) • • • In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: o It consists of a small core, or nucleus, that contains most of the mass of the atom o This nucleus is made up of particles called protons, which have a positive charge o The protons are surrounded by negatively charged electrons, but most of the atom is actually empty space Did extensive work on radioactivity (alpha & beta particles, gamma rays/waves) and was referred to as the “Father of Nuclear Physics” Won a Nobel Prize Tuesday, October 8, 13 Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Ernest Rutherford (1871 – 1937) • • • • In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: o It consists of a small core, or nucleus, that contains most of the mass of the atom o This nucleus is made up of particles called protons, which have a positive charge o The protons are surrounded by negatively charged electrons, but most of the atom is actually empty space Did extensive work on radioactivity (alpha & beta particles, gamma rays/waves) and was referred to as the “Father of Nuclear Physics” Won a Nobel Prize Was a student of J.J. Thomson Tuesday, October 8, 13 Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Ernest Rutherford (1871 – 1937) • • In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: o It consists of a small core, or nucleus, that contains most of the mass of the atom o This nucleus is made up of particles called protons, which have a positive charge o The protons are surrounded by negatively charged electrons, but most of the atom is actually empty space Did extensive work on radioactivity (alpha & beta particles, gamma rays/waves) and was referred to as the “Father of Nuclear Physics” • • Won a Nobel Prize Was a student of J.J. Thomson • Was on the New Zealand $100 bill Tuesday, October 8, 13 Image taken from: http:// www.scientific-web.com/en/Physics/ Biographies/ErnestRutherford.html Gold-Foil Experiment 19 Tuesday, October 8, 13 Niels Bohr (1885 – 1962) Tuesday, October 8, 13 Niels Bohr (1885 – 1962) Image taken from: commons.wikimedia.org/wiki/ File:Niels_Bohr.jpg Tuesday, October 8, 13 Niels Bohr (1885 – 1962) • In 1913, proposed the Bohr Model, which suggests that electrons travel around the nucleus of an atom in orbits or definite paths. Additionally, the electrons can jump from a path in one level to a path in another level (depending on their energy) Image taken from: commons.wikimedia.org/wiki/ File:Niels_Bohr.jpg Tuesday, October 8, 13 Niels Bohr (1885 – 1962) • In 1913, proposed the Bohr Model, which suggests that electrons travel around the nucleus of an atom in orbits or definite paths. Additionally, the electrons can jump from a path in one level to a path in another level (depending on their energy) • Won a Nobel Prize Image taken from: commons.wikimedia.org/wiki/ File:Niels_Bohr.jpg Tuesday, October 8, 13 Niels Bohr (1885 – 1962) • In 1913, proposed the Bohr Model, which suggests that electrons travel around the nucleus of an atom in orbits or definite paths. Additionally, the electrons can jump from a path in one level to a path in another level (depending on their energy) • Won a Nobel Prize Image taken from: commons.wikimedia.org/wiki/ File:Niels_Bohr.jpg • Worked with Ernest Rutherford Tuesday, October 8, 13 Quantum Mechanics Werner Heisenberg ~1926 γ Microscope Electron Tuesday, October 8, 13 Quantum Mechanics • Heisenberg Uncertainty Principle – Impossible to know both the velocity and position of an electron at the same time γ Microscope Electron Tuesday, October 8, 13 Werner Heisenberg ~1926 Quantum Mechanics • Heisenberg Uncertainty Principle – Impossible to know both the velocity and position of an electron at the same time γ Microscope Electron Tuesday, October 8, 13 Werner Heisenberg ~1926 Quantum Mechanics • Heisenberg Uncertainty Principle – Impossible to know both the velocity and position of an electron at the same time γ Microscope Electron Tuesday, October 8, 13 Werner Heisenberg ~1926 Erwin Schrodinger (1887-1961) Tuesday, October 8, 13 Erwin Schrodinger (1887-1961) Image taken from: nobelprize.org/.../1933/schrodingerbio.html Tuesday, October 8, 13 Erwin Schrodinger (1887-1961) • In 1926, he further explained the nature of electrons in an atom by stating that the exact location of an electron cannot be stated; therefore, it is more accurate to view the electrons in regions called electron clouds; electron clouds are places where the electrons are likely to be found Tuesday, October 8, 13 Image taken from: nobelprize.org/.../1933/schrodingerbio.html Erwin Schrodinger (1887-1961) • In 1926, he further explained the nature of electrons in an atom by stating that the exact location of an electron cannot be stated; therefore, it is more accurate to view the electrons in regions called electron clouds; electron clouds are places where the electrons are likely to be found • Did extensive work on the Wave formula Schrodinger equation Tuesday, October 8, 13 Image taken from: nobelprize.org/.../1933/schrodingerbio.html Erwin Schrodinger (1887-1961) • In 1926, he further explained the nature of electrons in an atom by stating that the exact location of an electron cannot be stated; therefore, it is more accurate to view the electrons in regions called electron clouds; electron clouds are places where the electrons are likely to be found • Did extensive work on the Wave formula Schrodinger equation • Won a Nobel Prize Tuesday, October 8, 13 Image taken from: nobelprize.org/.../1933/schrodingerbio.html James Chadwick (1891 – 1974) Tuesday, October 8, 13 James Chadwick (1891 – 1974) Image taken from: www.wired.com/.../news/2009/02/ dayintech_0227 Tuesday, October 8, 13 James Chadwick (1891 – 1974) • Realized that the atomic mass of most elements was double the number of protons discovery of the neutron in 1932 Image taken from: www.wired.com/.../news/2009/02/ dayintech_0227 Tuesday, October 8, 13 James Chadwick (1891 – 1974) • Realized that the atomic mass of most elements was double the number of protons discovery of the neutron in 1932 • Worked on the Manhattan Project Image taken from: www.wired.com/.../news/2009/02/ dayintech_0227 Tuesday, October 8, 13 James Chadwick (1891 – 1974) • Realized that the atomic mass of most elements was double the number of protons discovery of the neutron in 1932 • Worked on the Manhattan Project • Worked with Ernest Rutherford Image taken from: www.wired.com/.../news/2009/02/ dayintech_0227 Tuesday, October 8, 13 James Chadwick (1891 – 1974) • Realized that the atomic mass of most elements was double the number of protons discovery of the neutron in 1932 • Worked on the Manhattan Project • Worked with Ernest Rutherford • Won a Nobel Prize Image taken from: www.wired.com/.../news/2009/02/ dayintech_0227 Tuesday, October 8, 13 Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Democritus & John Dalton Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Progression of the Atomic Model - -- - + The structure of an atom, according to: Tuesday, October 8, 13 J.J. Thomson Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Ernest Rutherford Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Neils Bohr Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Erwin Schrodinger Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 Progression of the Atomic Model The structure of an atom, according to: Tuesday, October 8, 13 James Chadwick