TEST II Study Guide-Atomic Theory Honors Chemistry
... 6. ______ What type of bombarding particle was used in Rutherford's "gold foil" experiment? A) alpha; B) beta; C) gamma; D) neutron; E) neutrino; F) X rays. 7. ______ This color-blind English schoolteacher studied the findings of many of his contemporaries, and put forth the first comprehensive atom ...
... 6. ______ What type of bombarding particle was used in Rutherford's "gold foil" experiment? A) alpha; B) beta; C) gamma; D) neutron; E) neutrino; F) X rays. 7. ______ This color-blind English schoolteacher studied the findings of many of his contemporaries, and put forth the first comprehensive atom ...
chpt 11 and 12 notes with answers
... shells ◦ Mostly gases at room temperature ◦ “semiconductors” ◦ Border left side of “zigzag” ◦ Varying number of electrons in outer shell ◦ Share properties of both metals and nonmetals ...
... shells ◦ Mostly gases at room temperature ◦ “semiconductors” ◦ Border left side of “zigzag” ◦ Varying number of electrons in outer shell ◦ Share properties of both metals and nonmetals ...
File
... Definition: Compounds are formed when two or more different elements ___________________ combine. A ________________________ is the smallest physical unit of a substance that can exist independently, consisting of one or more atoms held together by chemical forces ____________________ ______________ ...
... Definition: Compounds are formed when two or more different elements ___________________ combine. A ________________________ is the smallest physical unit of a substance that can exist independently, consisting of one or more atoms held together by chemical forces ____________________ ______________ ...
Chemistry Final Study Guide
... Particles in gases are completely free to move and are always in motion. ...
... Particles in gases are completely free to move and are always in motion. ...
CP3
... of an isotope in amu’s is simply the Mass number Most elements have several common isotopes Mass on periodic table must reflect this, that is why there are decimals Weighted average calculation (like grades) ...
... of an isotope in amu’s is simply the Mass number Most elements have several common isotopes Mass on periodic table must reflect this, that is why there are decimals Weighted average calculation (like grades) ...
ATOM ATOMIC SYMBOL ATOMIC NUMBER
... Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass – Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) ...
... Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass – Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) ...
Atomic Theories during history
... published them in a book called "A New System of Chemical Philosophy. In it he was the first to propose that elements be identified with symbols. However, only 3 or 4 pages in the third chapter discussed the atomic theory he proposed. In this theory, there are four basic ideas... 1) chemical element ...
... published them in a book called "A New System of Chemical Philosophy. In it he was the first to propose that elements be identified with symbols. However, only 3 or 4 pages in the third chapter discussed the atomic theory he proposed. In this theory, there are four basic ideas... 1) chemical element ...
2008 Midterm Multiple Choice
... The characteristic bright-line spectrum of an element is produced when its electrons A) form an ionic bond B) return to a lower energy state C) move to a higher energy state D) form a covalent bond ...
... The characteristic bright-line spectrum of an element is produced when its electrons A) form an ionic bond B) return to a lower energy state C) move to a higher energy state D) form a covalent bond ...
Chapter 1
... B. Now for Some Neutrons C. Building Bigger Atoms D. Protons and Atomic Number *Notes-The number of protons in the nucleus of an atom give the element its _____________________. (also the number of electrons) ...
... B. Now for Some Neutrons C. Building Bigger Atoms D. Protons and Atomic Number *Notes-The number of protons in the nucleus of an atom give the element its _____________________. (also the number of electrons) ...
Chapter 4 Atomic Structure
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. Isotope ...
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. Isotope ...
Ch 2 Atoms and Molecules
... • The metals are seperated for nonmetals by a diagonal staircase line that runs from boron (B) to astatine (At). • Hydrogen, though a nonmetal, is on the left side of the periodic table. • The state of matter at which nonmetals are at room temperature, vary from element to element. ...
... • The metals are seperated for nonmetals by a diagonal staircase line that runs from boron (B) to astatine (At). • Hydrogen, though a nonmetal, is on the left side of the periodic table. • The state of matter at which nonmetals are at room temperature, vary from element to element. ...
CHEMISTRY EXAM 2 REVIEW
... Periodic Table, Physical and Chemical Properties, Changes, and Reactions Guardian Signature: _________________________________________________________________ My child completed this review and studied for at least 30 minutes. Define the following chemistry terms: [Chemistry Dictionary] 1. alloy a m ...
... Periodic Table, Physical and Chemical Properties, Changes, and Reactions Guardian Signature: _________________________________________________________________ My child completed this review and studied for at least 30 minutes. Define the following chemistry terms: [Chemistry Dictionary] 1. alloy a m ...
SOME BASIC CHEMICAL TERMS
... The ancient Greeks originated the concept that all matter is composed of a limited number of simple substances called elements. The Greeks assumed that all matter is derived from four elements: earth, air, fire, and water. Lavoisier firmly established the concept of a chemical element as a substance ...
... The ancient Greeks originated the concept that all matter is composed of a limited number of simple substances called elements. The Greeks assumed that all matter is derived from four elements: earth, air, fire, and water. Lavoisier firmly established the concept of a chemical element as a substance ...
elements and isotopes - vocabulary
... an isotope. atomic weight (relative atomic mass) The average mass of all atoms of a particular element found in nature. It is also called relative atomic mass. It is expressed in atomic mass units (amu). On the atomic mass scale, the mass of one atom of carbon-12 is set up as a standard and is exact ...
... an isotope. atomic weight (relative atomic mass) The average mass of all atoms of a particular element found in nature. It is also called relative atomic mass. It is expressed in atomic mass units (amu). On the atomic mass scale, the mass of one atom of carbon-12 is set up as a standard and is exact ...
Chapter 2: Chemistry of Life
... – Example – table salt – NaCl – sodium loses and electron and chlorine gains an electron ...
... – Example – table salt – NaCl – sodium loses and electron and chlorine gains an electron ...
Egyptian American International School Science Department Grade
... 3.1 The Elements VOCAB Element symbols Main Idea All of the materials in the universe can be chemically broken down into about 100 different elements. Nine elements account for about 98% of earth’s crust, oceans, and atmosphere. In the human body, oxygen, carbon, hydrogen, and nitrogen are the ...
... 3.1 The Elements VOCAB Element symbols Main Idea All of the materials in the universe can be chemically broken down into about 100 different elements. Nine elements account for about 98% of earth’s crust, oceans, and atmosphere. In the human body, oxygen, carbon, hydrogen, and nitrogen are the ...
honors_chapter_4
... If all atoms are made up the same particles, their masses should be simple multiples of each other. But, elements are made up of mixtures of two or more isotopes. The RAM reflects this. The RAM is the “weighted” average of the masses of the isotopes of an element. ...
... If all atoms are made up the same particles, their masses should be simple multiples of each other. But, elements are made up of mixtures of two or more isotopes. The RAM reflects this. The RAM is the “weighted” average of the masses of the isotopes of an element. ...
atoms = building blocks
... • Matter- the stuff that makes up everything in the universe • Element- A substance that cannot be broken down into other substances by chemical or physical means ...
... • Matter- the stuff that makes up everything in the universe • Element- A substance that cannot be broken down into other substances by chemical or physical means ...
Mileposts on the road to the atom (download)
... know how many atoms of one element combines with another Essential to know number of atoms to understand chemistry Atomic weight scale, enabled by Avogadro’s hypothesis, provides link between experimental observable (mass) and numbers of atoms ...
... know how many atoms of one element combines with another Essential to know number of atoms to understand chemistry Atomic weight scale, enabled by Avogadro’s hypothesis, provides link between experimental observable (mass) and numbers of atoms ...
Chapter 4 The Structure of the Atom
... Isotopes M atoms with the same number of protons and different number of neutrons M each isotope has essentially the same chemical properties because the protons and electrons are the same ...
... Isotopes M atoms with the same number of protons and different number of neutrons M each isotope has essentially the same chemical properties because the protons and electrons are the same ...
Atomic definitions
... The mass number of an atom is the total number of protons and neutrons in the nucleus of the atom. What about electrons? Don’t they count? Electrons are very light. It takes almost 2,000 electrons to equal the mass of one proton. Because of this, their mass is not counted in the atomic mass. Som ...
... The mass number of an atom is the total number of protons and neutrons in the nucleus of the atom. What about electrons? Don’t they count? Electrons are very light. It takes almost 2,000 electrons to equal the mass of one proton. Because of this, their mass is not counted in the atomic mass. Som ...
Atomic Mass
... same number of neutrons. •Isotopes are atoms of the same element with different numbers of neutrons. •Isotope symbol: ...
... same number of neutrons. •Isotopes are atoms of the same element with different numbers of neutrons. •Isotope symbol: ...
Document
... Chemical Changes and Properties of Matter • Chemical change or chemical reaction: • Making a NEW compound • The transformation of one or more atoms or molecules into one or more different molecules ...
... Chemical Changes and Properties of Matter • Chemical change or chemical reaction: • Making a NEW compound • The transformation of one or more atoms or molecules into one or more different molecules ...
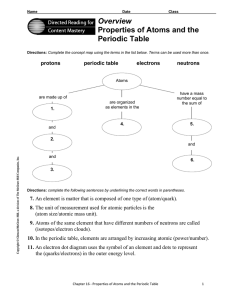
Overview Properties of Atoms and the Periodic Table
... number. The mass of the atom is so small that there is a ...
... number. The mass of the atom is so small that there is a ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.