Cellular Resp
... Flow of electrons along the chain accomplishes the active transport of protons (H+) across the inner mitochondrial membrane Protons diffuse back into the mitochondrial matrix through a proton channel which couples the diffusion to ATP synthesis ...
... Flow of electrons along the chain accomplishes the active transport of protons (H+) across the inner mitochondrial membrane Protons diffuse back into the mitochondrial matrix through a proton channel which couples the diffusion to ATP synthesis ...
Exam 2 for Review - philipdarrenjones.com
... 38) A patient has had a serious accident and lost a lot of blood. In an attempt to replenish body fluids, distilled water, equal to the volume of blood lost, is transferred directly into one of his veins. What will be the most probable result of this transfusion? A) It will have no unfavorable effec ...
... 38) A patient has had a serious accident and lost a lot of blood. In an attempt to replenish body fluids, distilled water, equal to the volume of blood lost, is transferred directly into one of his veins. What will be the most probable result of this transfusion? A) It will have no unfavorable effec ...
Chapter 10- Photosynthesis
... Photosynthesis also yields intermediates and products that can be used in lipid and amino acid synthesis. ...
... Photosynthesis also yields intermediates and products that can be used in lipid and amino acid synthesis. ...
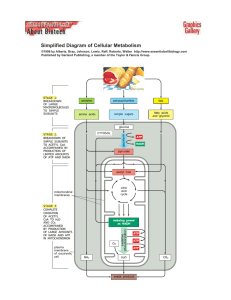
Simplified Diagram of Cellular Metabolism
... . http://www.essentialcellbiology.com Published by Garland Publishing, a member of the Taylor & Francis Group. ...
... . http://www.essentialcellbiology.com Published by Garland Publishing, a member of the Taylor & Francis Group. ...
Exam 4 key fall 2010
... Chemistry 160 Exam 4 Pg. 2 (5) 4. The balanced chemical equation for cellular respiration shows the use of oxygen. However, if you follow metabolic pathways, there is no oxygen consumed. Where is the oxygen? ...
... Chemistry 160 Exam 4 Pg. 2 (5) 4. The balanced chemical equation for cellular respiration shows the use of oxygen. However, if you follow metabolic pathways, there is no oxygen consumed. Where is the oxygen? ...
Lecture 20
... Pathways in eukaryotic cells occur in separate organelles or cellular locations ATP is made in the mitochondria and used in the cytosol. Fatty acids are make in the cytosol and broken down in the mitochondria. Separation of pathways exerts a greater control over opposing pathways and the intermedia ...
... Pathways in eukaryotic cells occur in separate organelles or cellular locations ATP is made in the mitochondria and used in the cytosol. Fatty acids are make in the cytosol and broken down in the mitochondria. Separation of pathways exerts a greater control over opposing pathways and the intermedia ...
Solomon chapter 8 practice AP bio test sept 2015
... Protons are pumped out of the mitochondria by the complexes of the electron transport chain. The proton gradient established during electron transport is a form of potential energy. The electron transport chain can be found in the mitochondria of aerobic bacteria and other cells. The movement of pro ...
... Protons are pumped out of the mitochondria by the complexes of the electron transport chain. The proton gradient established during electron transport is a form of potential energy. The electron transport chain can be found in the mitochondria of aerobic bacteria and other cells. The movement of pro ...
Who Wants To Be A Biologist?
... light-dependent reactions. This part of the light reactions makes ATP, which goes to the dark reactions to make glucose. ...
... light-dependent reactions. This part of the light reactions makes ATP, which goes to the dark reactions to make glucose. ...
Module code SB-2243 Module Title Introduction to Biochemistry
... and function of biologically important macromolecules and assemblies. It will also provide them with the concept of energy conservation and conversion processes in a living cell and thus lay a foundation in understanding the reactions of metabolism. Learning Outcomes ...
... and function of biologically important macromolecules and assemblies. It will also provide them with the concept of energy conservation and conversion processes in a living cell and thus lay a foundation in understanding the reactions of metabolism. Learning Outcomes ...
Cellular Respiration Harvesting Chemical Energy
... Structure of Mitochondria Mitochondria are found in almost all eukaryotic cells. Its structure is key to its role in cellular respiration. ...
... Structure of Mitochondria Mitochondria are found in almost all eukaryotic cells. Its structure is key to its role in cellular respiration. ...
2. What are the main properties that fats, proteins, and
... The primary function of pigments in plants is Photosynthesis, which uses the green pigment Chlorophyll along with several red and yellow pigments that help to capture as much light energy as possible. The chlorophyll pigments are located within the leaves. 31. What are the main products of the ligh ...
... The primary function of pigments in plants is Photosynthesis, which uses the green pigment Chlorophyll along with several red and yellow pigments that help to capture as much light energy as possible. The chlorophyll pigments are located within the leaves. 31. What are the main products of the ligh ...
Cell Respiration State that oxidation involves the loss of electrons
... TerminaI Oxidation and Oxidative PhosphoryIation In the Krebs cycle and glycolysis, pairs of hydrogen atoms are removed from the respiratory substrates. Oxidised NAD is converted into reduced NAD, except in the Krebs cycle, where FAD is reduced instead. Hydrogen atoms or their electrons are transpor ...
... TerminaI Oxidation and Oxidative PhosphoryIation In the Krebs cycle and glycolysis, pairs of hydrogen atoms are removed from the respiratory substrates. Oxidised NAD is converted into reduced NAD, except in the Krebs cycle, where FAD is reduced instead. Hydrogen atoms or their electrons are transpor ...
Study guide exam 1
... 27. List three factors that affect enzyme activity. 28. What are competitive and non-competitive inhibitors? 29. What are oxidation – reduction reactions? 30. What are the differences between catabolism and anabolism? 31. List three main ways that ATP is generated by. 32. What is carbohydrate metabo ...
... 27. List three factors that affect enzyme activity. 28. What are competitive and non-competitive inhibitors? 29. What are oxidation – reduction reactions? 30. What are the differences between catabolism and anabolism? 31. List three main ways that ATP is generated by. 32. What is carbohydrate metabo ...
Document
... 3. In general terms, explain the role of the electron transport chain in cellular respiration. 4. Identify the three stages of cellular respiration and state the region of the eukaryotic cell where each stage occurs. 5. Understand the process of glycolysis, and explain why ATP is required for the pr ...
... 3. In general terms, explain the role of the electron transport chain in cellular respiration. 4. Identify the three stages of cellular respiration and state the region of the eukaryotic cell where each stage occurs. 5. Understand the process of glycolysis, and explain why ATP is required for the pr ...
Review Questions for Advanced Biochemistry Course
... B. The production of oxaloacetate by pyruvate carboxylase is one of several anaplerotic reactions for the TCA cycle C. Succinyl CoA is used to create a neurotransmitter in the brain D. Pyruvate dehydrogenase helps convert pyruvate into malate E. Pyruvate carboxylase is only found in RBCs 32. Which o ...
... B. The production of oxaloacetate by pyruvate carboxylase is one of several anaplerotic reactions for the TCA cycle C. Succinyl CoA is used to create a neurotransmitter in the brain D. Pyruvate dehydrogenase helps convert pyruvate into malate E. Pyruvate carboxylase is only found in RBCs 32. Which o ...
Microbiology: A Systems Approach, 2nd ed.
... achieved by: – Increasing thermal energy to increase molecular velocity – Increasing the concentration of reactants to increase the rate of molecular collisions – Adding a catalyst ...
... achieved by: – Increasing thermal energy to increase molecular velocity – Increasing the concentration of reactants to increase the rate of molecular collisions – Adding a catalyst ...
Lorem Ipsum - Tri-County Technical College
... group is removed from amino acids The result is a keto acid Keto acids enter the respiratory cycle as pyruvic acid or as one of the other types of molecules found in the Kreb’s cycle. The amino group is converted to ammonia ...
... group is removed from amino acids The result is a keto acid Keto acids enter the respiratory cycle as pyruvic acid or as one of the other types of molecules found in the Kreb’s cycle. The amino group is converted to ammonia ...
Chapter 9
... lactic acid fermentation. • There are two types of anaerobes: facultative and obligate. • Facultative anaerobes can tolerate the presence of oxygen; they simply do ...
... lactic acid fermentation. • There are two types of anaerobes: facultative and obligate. • Facultative anaerobes can tolerate the presence of oxygen; they simply do ...
Document
... 1. Hydrogen ion “flow” down their gradient back into the inner compartment through ATP Synthase. 2. As they flow through the enzyme, it rotates (like a generator), and combines ADP + P (a phosphate group) and forms ATP! 3. The SPEED of the flow, POWERS the “recharging” of the ATP “battery”! ...
... 1. Hydrogen ion “flow” down their gradient back into the inner compartment through ATP Synthase. 2. As they flow through the enzyme, it rotates (like a generator), and combines ADP + P (a phosphate group) and forms ATP! 3. The SPEED of the flow, POWERS the “recharging” of the ATP “battery”! ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.