4.1 Section Assessment
... From his experiments, Rutherford concluded that an atom is made of a positively-charged nucleus surrounded by a region of empty space in which electrons orbit that nucleus. Rutherford believed that an atom’s nucleus was very tiny compared to the atom as a whole, and that, in spite of this, the nucle ...
... From his experiments, Rutherford concluded that an atom is made of a positively-charged nucleus surrounded by a region of empty space in which electrons orbit that nucleus. Rutherford believed that an atom’s nucleus was very tiny compared to the atom as a whole, and that, in spite of this, the nucle ...
AJAY PARMAR GROUP TUITION
... Experiments of Ernest Rutherford Rutherford presented the experiment to show how electrons are arranged in the atom. Rutherford produced alpha () rays from radioactive element polonium (Po). These rays were incident from one side on the foil (0.004 mm thick) of gold. Observations of Rutherford’s ...
... Experiments of Ernest Rutherford Rutherford presented the experiment to show how electrons are arranged in the atom. Rutherford produced alpha () rays from radioactive element polonium (Po). These rays were incident from one side on the foil (0.004 mm thick) of gold. Observations of Rutherford’s ...
Practice Packet Level 3: Atomics - Mr. Palermo`s Flipped Chemistry
... field. This suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson concluded that the atom was a positively charged sphere of almost uniform density in which ...
... field. This suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson concluded that the atom was a positively charged sphere of almost uniform density in which ...
Chem101 - Lecture 2 Elements Elements as Pure
... • The atomic mass unit is a very small unit of mass. • In the lab we typically use grams as our unit of mass. • A mole is defined as the number of atoms of an element who’s mass in grams is numerically equal to the atom’s mass in atomic mass units. ...
... • The atomic mass unit is a very small unit of mass. • In the lab we typically use grams as our unit of mass. • A mole is defined as the number of atoms of an element who’s mass in grams is numerically equal to the atom’s mass in atomic mass units. ...
5 Early Atomic Theory and Structure Chapter Outline Early Theories
... 3. Atoms of different elements differ in their mass and size. 4. Compounds are formed by combining two or more atoms of different elements. 5. Atoms combine to form compounds in simple whole number ratios. ...
... 3. Atoms of different elements differ in their mass and size. 4. Compounds are formed by combining two or more atoms of different elements. 5. Atoms combine to form compounds in simple whole number ratios. ...
Elements, Compounds, and Mixtures
... An element contains just one type of atom. A compound contains two or more different atoms joined together. A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds. ...
... An element contains just one type of atom. A compound contains two or more different atoms joined together. A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds. ...
- erc
... From all these studies it is observed that structure of the atom is same as the structure of the solar system. Centralley located protons and neutrons representing Sun whereas electrons revolving around the centre resembles planets. Shell proton ...
... From all these studies it is observed that structure of the atom is same as the structure of the solar system. Centralley located protons and neutrons representing Sun whereas electrons revolving around the centre resembles planets. Shell proton ...
Atoms and the Periodic Table
... 63 existing ELEMENTS in order by their increasing ATOMIC MASS number a. “PERIODIC” means having a regular, REPEATING pattern and it means a “listing” b. Mendeleev used the “periodic patterns” to PREDICT future ELEMENTS 4. In 1914, Moseley re-organized the PERIODIC Table according to each element’s ...
... 63 existing ELEMENTS in order by their increasing ATOMIC MASS number a. “PERIODIC” means having a regular, REPEATING pattern and it means a “listing” b. Mendeleev used the “periodic patterns” to PREDICT future ELEMENTS 4. In 1914, Moseley re-organized the PERIODIC Table according to each element’s ...
Redox Reactions - KFUPM Faculty List
... Oxidation-reduction reactions (sometimes called redox reactions)) are reactions involvingg the transfer of one electron or more from one reactant to another. Redox reaction also involves the change in oxidation states for molecules. These reactions are very common in life: • Photosynthesis. (convers ...
... Oxidation-reduction reactions (sometimes called redox reactions)) are reactions involvingg the transfer of one electron or more from one reactant to another. Redox reaction also involves the change in oxidation states for molecules. These reactions are very common in life: • Photosynthesis. (convers ...
Chapter 18 - Houston ISD
... cathode ray tubes, devices that were early versions of fluorescent and neon lights. Julius Plucker (18011868) and William Crooks, an English physicist and chemist (1832-1919), and his countryman and fellow physicist Joseph John Thomson (1856-1940) conducted many of these experiments. They showed tha ...
... cathode ray tubes, devices that were early versions of fluorescent and neon lights. Julius Plucker (18011868) and William Crooks, an English physicist and chemist (1832-1919), and his countryman and fellow physicist Joseph John Thomson (1856-1940) conducted many of these experiments. They showed tha ...
atomic regents review
... One model of the atom states that atoms are tiny particles composed of a uniform mixture of positive and negative charges. Scientists conducted an experiment where alpha particles were aimed at a thin layer of gold atoms. Most of the alpha particles passed directly through the gold atoms. A few alph ...
... One model of the atom states that atoms are tiny particles composed of a uniform mixture of positive and negative charges. Scientists conducted an experiment where alpha particles were aimed at a thin layer of gold atoms. Most of the alpha particles passed directly through the gold atoms. A few alph ...
Electron configuration
... Usually the excitation of valence electrons (such as 3s for sodium) involves energies corresponding to photons of visible or ultraviolet light. The excitation of core electrons is possible, but requires much higher energies generally corresponding to x-ray photons. This would be the case for example ...
... Usually the excitation of valence electrons (such as 3s for sodium) involves energies corresponding to photons of visible or ultraviolet light. The excitation of core electrons is possible, but requires much higher energies generally corresponding to x-ray photons. This would be the case for example ...
Chapter 18: Properties of Atoms and the Periodic Table
... The nucleus contains most of the mass of the atom because protons and neutrons are far more massive than electrons. The mass of a proton is about the same as that of a neutron—approximately 1.6726 ⫻ 10⫺24 g, as shown in Table 2. The mass of each is approximately 1,836 times greater than the mass of ...
... The nucleus contains most of the mass of the atom because protons and neutrons are far more massive than electrons. The mass of a proton is about the same as that of a neutron—approximately 1.6726 ⫻ 10⫺24 g, as shown in Table 2. The mass of each is approximately 1,836 times greater than the mass of ...
Atom
... kinds of atoms. _____________________ 3. Discovered radioactivity while working with a sample of Uranium. _____________________ 4. Determined that a cathode ray is made of electrons. _____________________ 5. Isolated two new elements, radium and polonium. _____________________ 6. Understood the atom ...
... kinds of atoms. _____________________ 3. Discovered radioactivity while working with a sample of Uranium. _____________________ 4. Determined that a cathode ray is made of electrons. _____________________ 5. Isolated two new elements, radium and polonium. _____________________ 6. Understood the atom ...
Adaptif Atomic Theory Rutherford
... number its(the proton and electron, and happened at different element. Isobar happened equality in atomic mass that is its(the proton amounts and neutron, but differs in every proton amounts, its(the electron and neutron, isobar happened at different element. Difference of one element to other eleme ...
... number its(the proton and electron, and happened at different element. Isobar happened equality in atomic mass that is its(the proton amounts and neutron, but differs in every proton amounts, its(the electron and neutron, isobar happened at different element. Difference of one element to other eleme ...
Chapter 18 - Powell County Schools
... English chemist and physicist John Dalton (1766-1844) was one of the first scientists to set out to gather evidence for the idea. Dalton was a remarkable person. Born into a family too poor to send him to school, young John educated himself and, at age 12, became a schoolteacher. He grew to be one o ...
... English chemist and physicist John Dalton (1766-1844) was one of the first scientists to set out to gather evidence for the idea. Dalton was a remarkable person. Born into a family too poor to send him to school, young John educated himself and, at age 12, became a schoolteacher. He grew to be one o ...
Families and Periods of the Periodic Table - CK
... The ten elements formed by filling in the 3d orbitals, as well as all other elements that have between 1 to 10 electrons in d orbitals, are called the transition elements. These elements, in general, differ from each other in the electron structure of the next-to-last energy level. For the most part ...
... The ten elements formed by filling in the 3d orbitals, as well as all other elements that have between 1 to 10 electrons in d orbitals, are called the transition elements. These elements, in general, differ from each other in the electron structure of the next-to-last energy level. For the most part ...
GLUCOSE - npd117.net
... atoms that The smallest unit in a are either compound (+) or (-) Na+ ClH2O ...
... atoms that The smallest unit in a are either compound (+) or (-) Na+ ClH2O ...
ISOTOPIC NOTATION isotopes are atoms with the same number
... PRACTICE PROBLEMS # 8 1. A sample of neon contains three isotopes, neon-20 (with an isotopic mass of 19.9924 amu), neon-21 (20.9939 amu) and neon-22 (21.9914 amu). The natural abundances of these isotopes are 90.92%, 0.257 %, and 8.82 %. Calculate the atomic weight of neon. ...
... PRACTICE PROBLEMS # 8 1. A sample of neon contains three isotopes, neon-20 (with an isotopic mass of 19.9924 amu), neon-21 (20.9939 amu) and neon-22 (21.9914 amu). The natural abundances of these isotopes are 90.92%, 0.257 %, and 8.82 %. Calculate the atomic weight of neon. ...
2.1 Imaging and Moving Individual Atoms
... experiment to measure the electronic charge. Drops of oil were carried past a uniform electric field between charged plates. After charging the drop with x-rays, he adjusted the electric field between the plates so that the oil drop was exactly balanced against the force of gravity. Then the charge ...
... experiment to measure the electronic charge. Drops of oil were carried past a uniform electric field between charged plates. After charging the drop with x-rays, he adjusted the electric field between the plates so that the oil drop was exactly balanced against the force of gravity. Then the charge ...
Atomic History and Structure:
... Hans G. and undergraduate Ernest M. worked for Rutherford.) “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scat ...
... Hans G. and undergraduate Ernest M. worked for Rutherford.) “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scat ...
Notes
... Draw a nucleus with the element symbol inside. Carbon is in the 2nd period, so it has two energy levels, or shells. ...
... Draw a nucleus with the element symbol inside. Carbon is in the 2nd period, so it has two energy levels, or shells. ...
6-2 Notes: The Atom
... because they are much smaller than an atom. The number of subatomic particles in an atom and the way they interact determine the _____________ of the atom. __________ are positively charged particles of the nucleus. The SI unit that describes the mass of a particle in an atom is the ________ ______ ...
... because they are much smaller than an atom. The number of subatomic particles in an atom and the way they interact determine the _____________ of the atom. __________ are positively charged particles of the nucleus. The SI unit that describes the mass of a particle in an atom is the ________ ______ ...
Slide 1
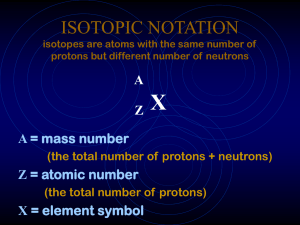
... • __________: atoms of the same element that have _______________ due to different numbers of __________. • _____________: the total number of _______ and _________ that make up the nucleus of an isotope ~ Isotopes are written with the _____________ written after the element name or symbol with a __ ...
... • __________: atoms of the same element that have _______________ due to different numbers of __________. • _____________: the total number of _______ and _________ that make up the nucleus of an isotope ~ Isotopes are written with the _____________ written after the element name or symbol with a __ ...