* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Transcript
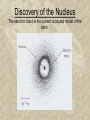
Chapter 3 The Atom The Atom • ____________ (450 B.C.) proposed that all matter is made up of tiny, indivisible particles. (atomos) 3-2 Laws • Law of Conservation of Mass: mass is neither ____________ during ordinary chemical rxns or ___________ changes. 3-3 Laws • Law of Definite Proportions: compounds contain the same ____________ in exactly the same _____________ by mass regardless of the _______ of the sample or the __________ of the compound. 3-4 Laws • Law of Multiple Proportions: If two or more ________________ are composed of the same ____________, the _______ of the masses of the second element combined with a certain _________ of the first element is always a ratio of ______________________. 3-5 Daltons Atomic Theory In 1808 ___________, an English schoolteacher, came up with an atomic theory to explain these laws. Many of the _________ of his theory still hold true today. 3-6 Dalton’s Atomic Theory ~ Each ____________ is composed of atoms. ~ Atoms of a given element are __________, and __________ than those of any other element. ~ A given compound forms by ____________ of two or more different atoms, always in the same _______________________ of atoms. ~ Atoms are neither ________________ in any chemical reaction, only _____________. 3-7 The Structure of the Atom • ______: the smallest _________ of an element that ________ the chemical ________ of that element. 3-8 The Discovery of the Electron • ___________ (1897) an English physicist who discovered ________ using his famous ___________ experiment. He determined the _______________ ratio. 3-9 The Discovery of the Electron The Discovery of the Electron • __________ (1909) an American __________ who determined the charge of electrons using his famous _________ experiment. The mass of an e- is approximately ________ the mass of an atom. 3-11 The Discovery of the Electron The Discovery of the Electron Based on these discoveries, two inferences were made: 1. Because atoms are ____________, they must contain a ________ charge to _________ the negative electrons. 2. Because e- are so light in mass _________ to atoms, atoms must contain other _____________ that account for most of its mass. 3-13 Plum Pudding Atomic Model Discovery of the Nucleus 1911, Ernest ________ conducted experiments with _________ materials that released only __________ charged alpha particles…. 3-15 Discovery of the Nucleus Discovery of the Nucleus • Rutherford concluded atoms have a ________ core with a _______ charge. Rutherford’s Atomic Model: Discovery of the Nucleus • Rutherford’s student, _________, came up with a way to explain the location of e- in the atom: Discovery of the Nucleus The electron cloud is the current accepted model of the atom. The Atom • Except for H, all ____________ contain _____________________. • A proton has a + charge ____________ _____________to the neg. charged e-. • Atoms contain _______________ of p+ and e-. • Neutrons are _____________ neutral. • P+ and no have almost ____________ masses, electrons weigh 1836 times ______. 3-20 The Atom • ______________: short range p+ to p+, p+ to no and no to no forces hold the nuclear particles together. • ___________________ (Z): the number of protons in each atom of a particular element. ☺The atomic number _________ the element! 3-21 Isotopes • __________: atoms of the same element that have _______________ due to different numbers of __________. • _____________: the total number of _______ and _________ that make up the nucleus of an isotope ~ Isotopes are written with the _____________ written after the element name or symbol with a _______: ex. Uranium-235 or U-235 3-22 Isotopes Uranium-235 or U-235 Mass number – atomic number = number of ________ 235 (protons + neutrons) – 92 protons = 143 neutrons This info could also be portrayed using a _____________: 235U 92 Isotopes ~ In nature, __________ are almost always found as a ___________ of isotopes ~ Isotopes have ________________ chemical properties ~Isotopes with _______ neutrons have a higher mass and are often described as “_______”. 3-24 Isotopes • Nuclide: a general term for a _________ __________ of an element. Atomic Mass • Atomic Mass Unit (____): one amu is exactly _____ of the mass of a ________ atom. • _______________: the weighted average of the atomic masses of the _________________ isotopes of an element. 3-26 Calculating the Average Moles • _____: the amount of a substance that contains as many _______ as there are atoms in exactly 12g of ___________. Moles • _____________: the number of particles in exactly one ______ of a pure substance ~ 6.02214179 x 1023 (we’ll use __________) Moles 602,000,000,00 0,000,000,000, 000 If you had 6.02 x 1023 pennies and gave away 1 million a day to every person on earth, it would take you 3000 years to distribute all your money!! 3-30 Molar Mass • ____________: the mass of one mole of a pure substance. ~ the mass of _________ atoms or __________ is measured in _____. The mass of a mole of the same substance is _____________ the same, with the units _______. Ex. H20 = H x 2 = 1.01 x 2 = 2.02 + O x 1 = 16.00 x 1 = +16.00 18.02 3-31 Molar Mass 1) What is the molar mass of BaCl2? 2) What is the molar mass of NaI? 1) Ba = _ x _____ g/mol = _______ Cl = _ x _____ g/mol = ______ _______ g/mol 2) Na = _ x ______ g/mol = ______ I = _ x _______ g/mol = _______ ________ g/mol 3-31 Molar Mass This photograph shows one mole of _______ (NaCl 58.44g/mol), ____ (H2O 18.02 g/mol), and ____(N2 28.02 g/mol). 3-33 Mass/Mole Conversions When given the number of _______, you can find the _______by: Moles x _g__ = grams mole Ex. 5.0 moles of H2O = X g 5.0 moles x 18.02g = 90. g H2O in 5.0 mol moles 3-34 Mass/Mole Conversions Moles x _g_ = moles mol Now try these problems: 3) 8.32 moles of barium chloride equals how many grams? 4) 20.1 moles of sulfur dioxide equals how many grams? 3-35 Mass/Mole Conversions 3) 8.32 moles of barium chloride equals how many grams? 8.32 moles BaCl2 x _____ g/mol = _____ grams BaCl2 4) 20.1 moles of sulfur dioxide equals how many grams? 20.1 moles SO2 x ______ g/mol = _____ g SO2 3-36 Mass/Mole Conversions Mass/Mole Conversions When given the amount in _______, you can calculate the number of ______ by: g x mol = moles g Ex. 11.2 g NaCl = X moles ___ g NaCl x 1.0 mol NaCl = _____ mols NaCl 58.44g 3-38 Mass/Mole Conversions g x mol = moles g Now try these problems: 5) 50.56 g of sodium chloride equals how many moles? 6) 329.8 g of ammonia equals how many moles? 3-39 Mass/Mole Conversions 5) 50.56 g of sodium chloride equals how many moles? _______ g NaCl x mole = _____ moles NaCl _____g 6) 329.8 g of ammonia equals how many moles? _____ g NH3 x mole = _____ moles NH3 ______g 3-40 Particle/Mole Conversions You can also calculate between _______ and number of ________: (1.0 moles = 6.02 x 1023 particles) To enter this number into your calculator, punch in 6.02 _____ button (________) 23. 3-41 Particle/Mole Conversions Ex. 2.59 moles of marble (CaCO3) contains how many molecules? _____ mol CaCO3 x 6.02 x 1023 molecules = ___________ 1.0 mol CaCO3 molecules *Particles can be molecules, atoms or formula units 7) How many molecules are in 5.0 moles of carbon dioxide? 5.0 mol CO2 x ________ molecules = 1.0 mole CO2 __________ molecules CO2 3-42 Particle/Mass Conversions Ex. What is the mass of 3.25 x1023 molecules of nitrogen? 3.25 x1023 N2 x ______ g = _____g N2 6.02 x 1023 8) How many molecules are 57.36 g of NaCl? 57.36 g NaCl x__________ = __________ ____ g molecules 3-43 The End!