Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
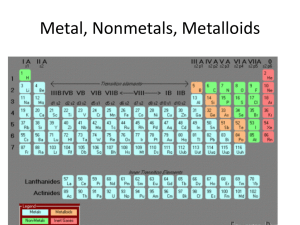
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
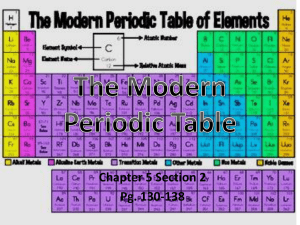
2.1 The Nature of Matter - Sonoma Valley High School
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
Worksheet
... 15. __________ He used the term “atomos” to describe an indivisible part at the base of all matter. 16. __________ He is the Father of the Atomic Theory. 17. __________ He designed a mathematical equation for the model of the atom. 18. __________ He determined the charge of the electron. 19. _______ ...
... 15. __________ He used the term “atomos” to describe an indivisible part at the base of all matter. 16. __________ He is the Father of the Atomic Theory. 17. __________ He designed a mathematical equation for the model of the atom. 18. __________ He determined the charge of the electron. 19. _______ ...
20161025140773
... – To have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard – An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom ...
... – To have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard – An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom ...
Chemical Basis of Life
... Title: The Chemical Basis of Life 1- Introduction: Your body is an elaborate chemical system. Chemical reactions power all of the body’s activities. At the most basic level, life is about chemicals and how they interact with each other. 2- Matter – Matter is anything that has mass and occupies space ...
... Title: The Chemical Basis of Life 1- Introduction: Your body is an elaborate chemical system. Chemical reactions power all of the body’s activities. At the most basic level, life is about chemicals and how they interact with each other. 2- Matter – Matter is anything that has mass and occupies space ...