Page 233 - ClassZone
... claimed that all matter was made of tiny particles he called atoms. Democritus said that all atoms were made of the same material. The objects of the world differed because each was made of atoms of different sizes and shapes. How does the modern view of atoms differ from this ancient view? How is i ...
... claimed that all matter was made of tiny particles he called atoms. Democritus said that all atoms were made of the same material. The objects of the world differed because each was made of atoms of different sizes and shapes. How does the modern view of atoms differ from this ancient view? How is i ...
Radioisotopes
... having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number of nucleons, the number of protons plus neutron ...
... having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number of nucleons, the number of protons plus neutron ...
Atomic Structure
... Electrons in the atom are to be found 90% of the time in 3D regions called orbitals THE BOHR ATOM When an electron transitions from the excited to the ground state, the atom loses energy When an electron transitions from the ground to the excited state, the atom absorbs energy Ground State Electron ...
... Electrons in the atom are to be found 90% of the time in 3D regions called orbitals THE BOHR ATOM When an electron transitions from the excited to the ground state, the atom loses energy When an electron transitions from the ground to the excited state, the atom absorbs energy Ground State Electron ...
Review 1st Qtr KEY
... CONCEPT QUESTIONS: Identify the letter of the choice that best completes the statement or answers the question. ANSWERS: A, B, B, B, B, B, C, C ____ 1. Most of the mass of an atom is found a. In the electron cloud. c. in the number of protons. b. in the nucleus. d. in the outer region of an atom. __ ...
... CONCEPT QUESTIONS: Identify the letter of the choice that best completes the statement or answers the question. ANSWERS: A, B, B, B, B, B, C, C ____ 1. Most of the mass of an atom is found a. In the electron cloud. c. in the number of protons. b. in the nucleus. d. in the outer region of an atom. __ ...
Introduction to the Periodic Table
... The number of protons and neutrons in the nucleus of an atom. ...
... The number of protons and neutrons in the nucleus of an atom. ...
Chapter 4 Study Guide Physical Science 1. The word atom comes
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
Atomic Structure and the Periodic Table
... • Electron cloud – cloud that surrounds the nucleus of an atom that describes the region in which an electron is most likely to be. – Example: students in a school ...
... • Electron cloud – cloud that surrounds the nucleus of an atom that describes the region in which an electron is most likely to be. – Example: students in a school ...
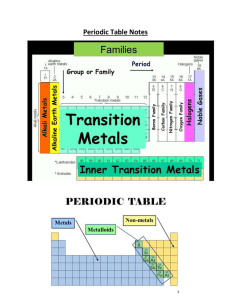
Chapter 6 Review“The Periodic Table”
... 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What does the number 84 represe ...
... 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What does the number 84 represe ...
Setting up Programmable PRS Keypad as Fixed ID Keypads
... Parser06 example6
The identity of an element is determined by the number of…
Q
Protons
Neutrons
Electrons
A molecule may consist of one atom.
Q
True
False
Molybdenum has atomic number 42. Its molar mass is 95.94 grams/mole. How many neutrons does
the most common isotope have?
Q
...
... Parser06 example
Learning Standards vocab chemical basis and molecules of life 09
... Explain the meaning of a chemical formula for an ionic array (e.g., NaCl). Give examples to illustrate that molecules are groups of two or more atoms bonded together (e.g., a molecule of water is formed when one oxygen atom shares electrons with two hydrogen atoms). Explain the meaning of a ch ...
... Explain the meaning of a chemical formula for an ionic array (e.g., NaCl). Give examples to illustrate that molecules are groups of two or more atoms bonded together (e.g., a molecule of water is formed when one oxygen atom shares electrons with two hydrogen atoms). Explain the meaning of a ch ...
Study Guide - Honors Chemistry
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
Intro to Atoms Clicker Questions 1. "atomos" means? 2. Atoms of one
... 2. Atoms of one kind of element _______ be changed into a different element with ordinary chemical means. (can, can’t) 3. Every compound is composed of atoms of different elements combined how? 4. In Thompson's model of the atom, the negatively charged electrons were located how in the atom? 5. In R ...
... 2. Atoms of one kind of element _______ be changed into a different element with ordinary chemical means. (can, can’t) 3. Every compound is composed of atoms of different elements combined how? 4. In Thompson's model of the atom, the negatively charged electrons were located how in the atom? 5. In R ...
Properties of matter student notes[1]
... Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
... Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
Review: Atomic structure/Periodic Table
... Formulas: not provided on the test, you should know them. Also, you can not use a graphing calculator on this test. If you want/need a calculator, bring a non-graphing one. ...
... Formulas: not provided on the test, you should know them. Also, you can not use a graphing calculator on this test. If you want/need a calculator, bring a non-graphing one. ...
– Units 5-7 Review Honors Chemistry Unit 5
... How many electrons does a sulfide ion have? How many protons, neutrons, and electrons are in Nickel-58? The element copper is found to contain the naturally occurring isotopes 29Cu63 and 29Cu65. The relative abundances are 69.1% and 30.9% respectively. Calculate the average atomic mass of copper. Ni ...
... How many electrons does a sulfide ion have? How many protons, neutrons, and electrons are in Nickel-58? The element copper is found to contain the naturally occurring isotopes 29Cu63 and 29Cu65. The relative abundances are 69.1% and 30.9% respectively. Calculate the average atomic mass of copper. Ni ...
L.O.
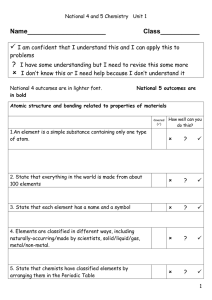
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
NOTES: 2.1 - Intro to Chemistry
... Isotopes: atoms of an element that have different # of neutrons ● in nature, elements occur as mixtures of isotopes ● some are radioactive: unstable isotope where nucleus decays emitting subatomic particles and/or energy as radioactivity causing one element to transform into another element ...
... Isotopes: atoms of an element that have different # of neutrons ● in nature, elements occur as mixtures of isotopes ● some are radioactive: unstable isotope where nucleus decays emitting subatomic particles and/or energy as radioactivity causing one element to transform into another element ...
Learning Targets
... 1. State the location and charges of each subatomic particle within the atom 2. Use the periodic table to determine the number of protons, neutrons, and electrons in an atom 3. Define atomic number as it relates to subatomic particles 4. Define atomic mass as it relates to subatomic particles. 5. Dr ...
... 1. State the location and charges of each subatomic particle within the atom 2. Use the periodic table to determine the number of protons, neutrons, and electrons in an atom 3. Define atomic number as it relates to subatomic particles 4. Define atomic mass as it relates to subatomic particles. 5. Dr ...
Period Table, valence Electrons and Ion Notes
... Example: Na = 1s2 2s2 2p6 3s1 Add up the e-‘s found in the last energy level, in this case there is only 1 so Na has 1 valence e**You have to do this for the Transition metal every time** ...
... Example: Na = 1s2 2s2 2p6 3s1 Add up the e-‘s found in the last energy level, in this case there is only 1 so Na has 1 valence e**You have to do this for the Transition metal every time** ...
atomic number - Thomas C. Cario Middle School
... The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom. The atomic number of an atom is equal to the number of protons in its nucleus. The number of electrons surrounding the nucleus of an atom is equal ...
... The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom. The atomic number of an atom is equal to the number of protons in its nucleus. The number of electrons surrounding the nucleus of an atom is equal ...













![Properties of matter student notes[1]](http://s1.studyres.com/store/data/009076956_1-3293fc3fecf578fd34e3f0f2700d471f-300x300.png)