Atomic Structure AKS Correlation Use the modern atomic theory to
... FILL OUT THE CHART BELOW. Radiation type ...
... FILL OUT THE CHART BELOW. Radiation type ...
Periodic Table Vocabulary Periodic Table – a chart that organizes
... The Law of Conservation of Matter – a scientific law that states that during a chemical reaction, matter cannot be created or destroyed but can be changed into a different form. Period law- The chemical properties of elements tends to repeat over specific atomic number intervals Law of definite prop ...
... The Law of Conservation of Matter – a scientific law that states that during a chemical reaction, matter cannot be created or destroyed but can be changed into a different form. Period law- The chemical properties of elements tends to repeat over specific atomic number intervals Law of definite prop ...
Ch. 4: Atoms and the Periodic Table – Study Guide
... Ionization refers to the process of losing or gaining electrons. The valence electron of a lithium atom is easily removed to form a lithium ion with a charge of 1+. A lithium ion is much less reactive than a lithium atom because it has a full outermost energy level. Isotopes of an element have the s ...
... Ionization refers to the process of losing or gaining electrons. The valence electron of a lithium atom is easily removed to form a lithium ion with a charge of 1+. A lithium ion is much less reactive than a lithium atom because it has a full outermost energy level. Isotopes of an element have the s ...
Atoms, Bonding, and the Periodic Table Electron Dot Diagrams
... Electron Dot Diagrams The valence electrons of an atom are shown as dots around the symbol of the element. Complete the electron dot diagram for neon. ...
... Electron Dot Diagrams The valence electrons of an atom are shown as dots around the symbol of the element. Complete the electron dot diagram for neon. ...
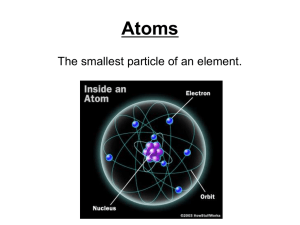
Atom The smallest piece of matter that still has the properties of the
... ForceForce Weak Nuclear Force Strong Nuclear Force Periodic Tablee ...
... ForceForce Weak Nuclear Force Strong Nuclear Force Periodic Tablee ...
Review Notes - Biochemistry
... 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged and when an electron is lost it will be _POSITIVE_ charged. ...
... 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged and when an electron is lost it will be _POSITIVE_ charged. ...
Unit 2 Overview
... in elements. After reviewing the basic structure of the atom, we will review some of the key experiments that lead to our current understanding of atomic structure. This unit is divided into three parts, the proton, the neutron, and the electron. In part one, we will take a closer look at how the nu ...
... in elements. After reviewing the basic structure of the atom, we will review some of the key experiments that lead to our current understanding of atomic structure. This unit is divided into three parts, the proton, the neutron, and the electron. In part one, we will take a closer look at how the nu ...
Salesian High School Elements and atoms Chemistry quiz The
... 7) Which of the following is one of the statements that make up Dalton’s atomic theory? a) All atoms contain electrons. b) All atoms of a given element are identical. c) Atoms are divisible. d) Atoms gain and lose electrons in chemical reactions ...
... 7) Which of the following is one of the statements that make up Dalton’s atomic theory? a) All atoms contain electrons. b) All atoms of a given element are identical. c) Atoms are divisible. d) Atoms gain and lose electrons in chemical reactions ...
BellWork 2/16/2015
... In an isotope, the number of protons and electrons never changes- only the number of neutrons is different This means that each isotope of a particular element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of ...
... In an isotope, the number of protons and electrons never changes- only the number of neutrons is different This means that each isotope of a particular element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of ...
IPC Atoms and Periodic Table
... of the naturally occurring isotopes of an element • Reported as atomic mass on the periodic ...
... of the naturally occurring isotopes of an element • Reported as atomic mass on the periodic ...
Practice Test #2 - smhs
... Answer the following questions based on the -2 anion of an isotopic form of sulfur: S-35 24.________ What is the A number for this nuclide? 25.________ What is the Z number for this nuclide? 26.________ What is the number of protons in the anion form of this nonmetal? 27.________ What is the number ...
... Answer the following questions based on the -2 anion of an isotopic form of sulfur: S-35 24.________ What is the A number for this nuclide? 25.________ What is the Z number for this nuclide? 26.________ What is the number of protons in the anion form of this nonmetal? 27.________ What is the number ...
Physical Science Chapter 6 Study Guide Atomic Theory of Matter
... o Mercury is the only metal that is liquid at room temperature o Oxygen makes of a majority of the mass of the human body ...
... o Mercury is the only metal that is liquid at room temperature o Oxygen makes of a majority of the mass of the human body ...
17 review for test - Blair Community Schools
... What is the atomic mass? What determines that identity of an atom? What happens to metallic properties as one goes across the table? ...
... What is the atomic mass? What determines that identity of an atom? What happens to metallic properties as one goes across the table? ...
Atomic Structure
... 19. Lead has 4 naturally occurring isotopes. Listed below are symbols for these isotopes along with their percent abundance. Using this information, calculate the “average” atomic mass of Pb. (Note: You may complete this calculation on the back of a one of the sheets in this packet). 122 Pb ...
... 19. Lead has 4 naturally occurring isotopes. Listed below are symbols for these isotopes along with their percent abundance. Using this information, calculate the “average” atomic mass of Pb. (Note: You may complete this calculation on the back of a one of the sheets in this packet). 122 Pb ...
atomic structure - IGCSE STUDY BANK
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
Quiz: The Atom (Open Notes)
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
1 - cloudfront.net
... How is the number of neutrons in the nucleus of an atom calculated? All atoms are neutral, with the number of protons equaling the ___. Isotopes of the same element have different _____. Using the periodic table, determine the number of neutrons in 16O. What does the number 84 represent in the name ...
... How is the number of neutrons in the nucleus of an atom calculated? All atoms are neutral, with the number of protons equaling the ___. Isotopes of the same element have different _____. Using the periodic table, determine the number of neutrons in 16O. What does the number 84 represent in the name ...
C2- Topic 1: Atomic structure and the periodic table. Assessable
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
C2 Topic 1 Can Do Sheet
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
Chemistry lecture notes
... Gamma radiation, is high energy electro magnetic radiation accompanies alpha and beta decays. Half-life is the time taken for half of a sample to decay. It can vary from a fraction of a second to billions of years. All elements beyond Bi (z=83) are radioactive and non beyond U(z=92) occur natural ...
... Gamma radiation, is high energy electro magnetic radiation accompanies alpha and beta decays. Half-life is the time taken for half of a sample to decay. It can vary from a fraction of a second to billions of years. All elements beyond Bi (z=83) are radioactive and non beyond U(z=92) occur natural ...
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... the density of gold in g/cm3 to the correct number of significant figures. ...
... the density of gold in g/cm3 to the correct number of significant figures. ...
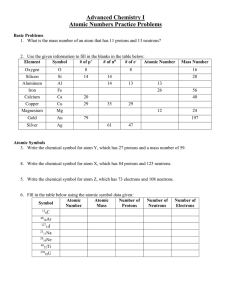
Atomic Numbers Practice Problems
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
Chapter 18 Test Review
... What is the mass number of an atom? ◦ What units are used to measure atomic mass? ...
... What is the mass number of an atom? ◦ What units are used to measure atomic mass? ...
Isotopes and Ions - Wando High School
... Ions IONS are charged atoms (or groups of atoms) that have a ...
... Ions IONS are charged atoms (or groups of atoms) that have a ...