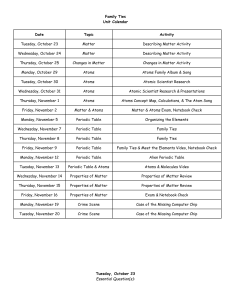
Ionic Bond - hrsbstaff.ednet.ns.ca
... • The outer most electron shell in any atom is called the valence shell • The electrons in the valence shell are called Valence Electrons • By looking at the number of valence electrons an element has we can predict its reactivity. • THE OCTET RULE: Atoms will try to lose, gain or share electrons to ...
... • The outer most electron shell in any atom is called the valence shell • The electrons in the valence shell are called Valence Electrons • By looking at the number of valence electrons an element has we can predict its reactivity. • THE OCTET RULE: Atoms will try to lose, gain or share electrons to ...
File
... have both metallic and nonmetallic properties. The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. The elements in the first period o ...
... have both metallic and nonmetallic properties. The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. The elements in the first period o ...
Up And Atom - Lesson Corner
... element are given in each square of the Periodic table: atomic number, atomic mass, chemical symbol and name. The number of protons in an element determines its properties. The number at the top of each square in the table tells how many protons the element has; the atomic number is unique to that e ...
... element are given in each square of the Periodic table: atomic number, atomic mass, chemical symbol and name. The number of protons in an element determines its properties. The number at the top of each square in the table tells how many protons the element has; the atomic number is unique to that e ...
PSI AP CHEMISTRY Atomic Theory and Models of the Atom Classwork:
... 1. An element is found to gain three electrons when it forms an ion. a) What group number would this element be found in? b) Is there enough information provided to determine what period it is in? 2. Look at the average atomic mass of Ar and K. a) Explain why early scientists might have been tempted ...
... 1. An element is found to gain three electrons when it forms an ion. a) What group number would this element be found in? b) Is there enough information provided to determine what period it is in? 2. Look at the average atomic mass of Ar and K. a) Explain why early scientists might have been tempted ...
PSI AP CHEMISTRY Summer Assignment Review Unit Free
... 1. An element is found to gain three electrons when it forms an ion. a) What group number would this element be found in? b) Is there enough information provided to determine what period it is in? 2. Look at the average atomic mass of Ar and K. a) Explain why early scientists might have been tempted ...
... 1. An element is found to gain three electrons when it forms an ion. a) What group number would this element be found in? b) Is there enough information provided to determine what period it is in? 2. Look at the average atomic mass of Ar and K. a) Explain why early scientists might have been tempted ...
Atomic Theoryx
... • -all matter is made of atoms • -atoms of one element are identical, atoms of different elements are different • -atoms form compounds in simple whole number ratios • -chemical reactions are rearrangements of atoms, the atoms are not changed • -compounds with different ratios of atoms are different ...
... • -all matter is made of atoms • -atoms of one element are identical, atoms of different elements are different • -atoms form compounds in simple whole number ratios • -chemical reactions are rearrangements of atoms, the atoms are not changed • -compounds with different ratios of atoms are different ...
6.022 X 10 23 atoms - Fort Thomas Independent Schools
... chemical properties. Atoms of different elements have different physical and chemical properties. ...
... chemical properties. Atoms of different elements have different physical and chemical properties. ...
Jeopardy - My CCSD
... An example of this ‘mixture of metals’ is bronze, which is created by combining copper and tin. ...
... An example of this ‘mixture of metals’ is bronze, which is created by combining copper and tin. ...
ch03 - earthjay science
... geochronology (29): The study of time as applied to Earth and planetary history. half-life (39): The time in which one-half of an original amount of a radioactive atoms decays to daughter products. Holocene Series (34): A term sometimes used to designate the period of time since the last major episo ...
... geochronology (29): The study of time as applied to Earth and planetary history. half-life (39): The time in which one-half of an original amount of a radioactive atoms decays to daughter products. Holocene Series (34): A term sometimes used to designate the period of time since the last major episo ...
Period:______ Table Number
... 46. The smallest particle of any element that you can have which still possesses all of the physical and chemical properties of that element is a single ATOM of that element. P. 10, VCR: Atoms and Molecules 47. Nearly 2000 years ago the Greek philosopher DEMOCRITUS gave us the word atom when he said ...
... 46. The smallest particle of any element that you can have which still possesses all of the physical and chemical properties of that element is a single ATOM of that element. P. 10, VCR: Atoms and Molecules 47. Nearly 2000 years ago the Greek philosopher DEMOCRITUS gave us the word atom when he said ...
Chapter 3—Time and Geology
... geochronology (27): The study of time as applied to Earth and planetary history. half-life (38): The time in which one-half of an original amount of a radioactive atoms decays to daughter products. Holocene Series (32): A term sometimes used to designate the period of time since the last major episo ...
... geochronology (27): The study of time as applied to Earth and planetary history. half-life (38): The time in which one-half of an original amount of a radioactive atoms decays to daughter products. Holocene Series (32): A term sometimes used to designate the period of time since the last major episo ...
Name: Date:______ Period:____ Isotopes and Atomic Mass
... The name atom comes from the Greek word "ἄτομος"—átomos, which means uncuttable or indivisible, something that cannot be divided further. The concept of an atom as an indivisible component of matter was first proposed by early Indian and Greek philosophers. In the 17th and 18th centuries, chemists p ...
... The name atom comes from the Greek word "ἄτομος"—átomos, which means uncuttable or indivisible, something that cannot be divided further. The concept of an atom as an indivisible component of matter was first proposed by early Indian and Greek philosophers. In the 17th and 18th centuries, chemists p ...
ch14 lecture 7e
... Zeff increases for the larger 3A elements due to poor shielding by d and f electrons. The larger 3A elements have smaller atomic radii and larger ionization energies and electronegativities than expected. These properties influence the physical and chemical behavior of these elements. ...
... Zeff increases for the larger 3A elements due to poor shielding by d and f electrons. The larger 3A elements have smaller atomic radii and larger ionization energies and electronegativities than expected. These properties influence the physical and chemical behavior of these elements. ...
5.3.1 The Nuclear Atom
... How did Rutherford interpret the results? Rutherford had expected all the alpha radiation to pass through the gold foil. He was surprised that some alpha particles were deflected slightly or bounced back. The ‘plum pudding’ model could not explain these results, so Rutherford proposed his ‘nuclear’ ...
... How did Rutherford interpret the results? Rutherford had expected all the alpha radiation to pass through the gold foil. He was surprised that some alpha particles were deflected slightly or bounced back. The ‘plum pudding’ model could not explain these results, so Rutherford proposed his ‘nuclear’ ...
Chemical Change
... of another subatomic particle. Neutron – subatomic particles with no charge but with a mass nearly equal to that of a proton. ...
... of another subatomic particle. Neutron – subatomic particles with no charge but with a mass nearly equal to that of a proton. ...
Mass Number, A
... the four elements: • earth (cold and dry) • air (hot and moist) • fire (hot and dry) • water (cold and moist) • The differences in matter where a result of different balances of these atoms • Changing the balance could change matter • ex: what we know as ...
... the four elements: • earth (cold and dry) • air (hot and moist) • fire (hot and dry) • water (cold and moist) • The differences in matter where a result of different balances of these atoms • Changing the balance could change matter • ex: what we know as ...
Excerpt - Assets - Cambridge
... which could not be divided further or destroyed, and (ii) all atoms of the same element were identical. This model was very helpful, but gave way to better models, as science and technology produced new evidence. This evidence has shown scientists that atoms have other particles inside them – they h ...
... which could not be divided further or destroyed, and (ii) all atoms of the same element were identical. This model was very helpful, but gave way to better models, as science and technology produced new evidence. This evidence has shown scientists that atoms have other particles inside them – they h ...
,ALgor (JoWr z:
... Q5 C The atomic number of sulfur is 16; it is in group 6 and therefore has six valence electrons. ...
... Q5 C The atomic number of sulfur is 16; it is in group 6 and therefore has six valence electrons. ...
Chapter 2
... • Valence electrons are those in the outermost shell, or valence shell • The chemical behavior of an atom is mostly determined by the valence electrons • Elements with a full valence shell are chemically inert • The Octet Rule: Rule that a valence shell is complete when it contains eight electrons e ...
... • Valence electrons are those in the outermost shell, or valence shell • The chemical behavior of an atom is mostly determined by the valence electrons • Elements with a full valence shell are chemically inert • The Octet Rule: Rule that a valence shell is complete when it contains eight electrons e ...
Atomic Structure PowerPoint Presentation
... Law of Multiple Proportions o The Law of Multiple Proportions states that atoms of two or more elements may combine in different ratios to produce more than one compound ...
... Law of Multiple Proportions o The Law of Multiple Proportions states that atoms of two or more elements may combine in different ratios to produce more than one compound ...