oxidative phosphorylation
... The matrix of mitochondrion is filled with dense fluid which is formed of fine granular substance as observed under electron microscope. 1- About 50% of the matrix content is formed of proteins; enzymes that are responsible for: a) Degradation of fatty acids and pyruvate to acetyl Co–enzyme A. b) Ox ...
... The matrix of mitochondrion is filled with dense fluid which is formed of fine granular substance as observed under electron microscope. 1- About 50% of the matrix content is formed of proteins; enzymes that are responsible for: a) Degradation of fatty acids and pyruvate to acetyl Co–enzyme A. b) Ox ...
Metabolism: Citric acid cycle
... 18. An anaplerotic reaction (of Greek origin, meaning to "fill up") is a reaction that leads to the net synthesis, or replenishment, of pathway components. A. Do mammals need this reaction to replenish metabolites of the citric acid cycle? ...
... 18. An anaplerotic reaction (of Greek origin, meaning to "fill up") is a reaction that leads to the net synthesis, or replenishment, of pathway components. A. Do mammals need this reaction to replenish metabolites of the citric acid cycle? ...
General Chemistry 110 Quiz 1
... PROCEEDING TO THE SHORT ANSWER SECTION. Number 1 through 15 are worth 6 points each. ...
... PROCEEDING TO THE SHORT ANSWER SECTION. Number 1 through 15 are worth 6 points each. ...
Microbial Metabolism
... joins with Oxaloacetic Acid (4C) to make Citric Acid (6C) Citric acid is oxidized releasing CO2 , free H+, & e- and forming ketoglutaric acid (5C) Free e- reduce the energy carriers NAD+ to NADH and FAD+ to FADH2 Ketoglutaric acid is also oxidized releasing more CO2 , free H+, & eThe cycle continues ...
... joins with Oxaloacetic Acid (4C) to make Citric Acid (6C) Citric acid is oxidized releasing CO2 , free H+, & e- and forming ketoglutaric acid (5C) Free e- reduce the energy carriers NAD+ to NADH and FAD+ to FADH2 Ketoglutaric acid is also oxidized releasing more CO2 , free H+, & eThe cycle continues ...
Slide 1
... transformed to pyruvate with production of a small amount of energy in the form of ATP or NADH. Glycolysis is an anaerobic process (it does not require oxygen). Glycolysis pathway is used by anaerobic as well as aerobic organisms. In glycolysis one molecule of glucose is converted into two molecules ...
... transformed to pyruvate with production of a small amount of energy in the form of ATP or NADH. Glycolysis is an anaerobic process (it does not require oxygen). Glycolysis pathway is used by anaerobic as well as aerobic organisms. In glycolysis one molecule of glucose is converted into two molecules ...
Overview of metabolism
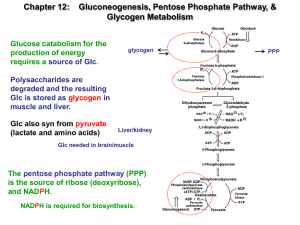
... formed from non-carbohydrate sources for survival. It also occurs during intense exercise. These non-carbohydrate precursors include lactate, pyruvate, propionate, glycerol (from diet and lipolysis) and glucogenic amino acids. Site: Mitochondria and cytosol of Liver and kidney are almost the only or ...
... formed from non-carbohydrate sources for survival. It also occurs during intense exercise. These non-carbohydrate precursors include lactate, pyruvate, propionate, glycerol (from diet and lipolysis) and glucogenic amino acids. Site: Mitochondria and cytosol of Liver and kidney are almost the only or ...
Chemistry of Life Review Sheet Key
... ionic bond- formed when 2 ions are attracted to one another. covalent bond- formed when atoms share electron(s). polar molecule- molecule with positive and negative ends hydrogen bond- formed between hydrogen of 1 molecule and negative end (usually oxygen) of another molecule. dehydration synthesis ...
... ionic bond- formed when 2 ions are attracted to one another. covalent bond- formed when atoms share electron(s). polar molecule- molecule with positive and negative ends hydrogen bond- formed between hydrogen of 1 molecule and negative end (usually oxygen) of another molecule. dehydration synthesis ...
Enzymes
... • Chemical chaos would result if a cell’s metabolic pathways were not tightly regulated • To regulate metabolic pathways, the cell switches on or off the genes that encode specific enzymes ...
... • Chemical chaos would result if a cell’s metabolic pathways were not tightly regulated • To regulate metabolic pathways, the cell switches on or off the genes that encode specific enzymes ...
Xe + Y → X + Ye - Sonoma Valley High School
... 24. Oxidative phosphorylation involves two components: the electron transport chain and ATP synthesis. Referring to Figure 9.13, notice that each member of the electron transport chain is lower in free __________ than the preceding member of the chain, but higher in _______________. The molecule at ...
... 24. Oxidative phosphorylation involves two components: the electron transport chain and ATP synthesis. Referring to Figure 9.13, notice that each member of the electron transport chain is lower in free __________ than the preceding member of the chain, but higher in _______________. The molecule at ...
Chapter 13 Carbohydrate Metabolism
... to glucose-6-phosphate and initiates the glycolysis pathway. • The enzyme is inhibited by a high concentration of glucose-6-phosphate (feedback inhibition). – phosphofructokinase — catalyzes the irreversible conversion of fructose 6-phosphate to fructose 1,6bisphosphate • As an allosteric enzyme, it ...
... to glucose-6-phosphate and initiates the glycolysis pathway. • The enzyme is inhibited by a high concentration of glucose-6-phosphate (feedback inhibition). – phosphofructokinase — catalyzes the irreversible conversion of fructose 6-phosphate to fructose 1,6bisphosphate • As an allosteric enzyme, it ...
Carbohydrate and sugar structure
... When close to equilibrium, [C][D]/[A][B]Keq and DG 0. This is true for many metabolic reactions – near-equilibrium reactions When reactants are in excess, the reaction shifts toward products When product are in excess, the reaction shifts toward reactants However, some reactions are not near equi ...
... When close to equilibrium, [C][D]/[A][B]Keq and DG 0. This is true for many metabolic reactions – near-equilibrium reactions When reactants are in excess, the reaction shifts toward products When product are in excess, the reaction shifts toward reactants However, some reactions are not near equi ...
Note 4.2 - Aerobic Respiration
... electron and proton acceptor is oxygen (O2). This process is made up of four protein complexes, which release energy in a series of stages, as to not damage the cell with an increase in temperature. Phase of the Electron Transport Chain: a) ...
... electron and proton acceptor is oxygen (O2). This process is made up of four protein complexes, which release energy in a series of stages, as to not damage the cell with an increase in temperature. Phase of the Electron Transport Chain: a) ...
Friday`s presentation.
... The theory of chemiosmotic coupling explains how the concentration gradient of H+ is used to generate energy to make ATP. a. The enzyme complex ATP synthase synthesizes ATP using the energy stored in the concentration gradient of H+ ions (i.e., protons) across the inner membrane, which is relatively ...
... The theory of chemiosmotic coupling explains how the concentration gradient of H+ is used to generate energy to make ATP. a. The enzyme complex ATP synthase synthesizes ATP using the energy stored in the concentration gradient of H+ ions (i.e., protons) across the inner membrane, which is relatively ...
Slide 1
... The theory of chemiosmotic coupling explains how the concentration gradient of H+ is used to generate energy to make ATP. a. The enzyme complex ATP synthase synthesizes ATP using the energy stored in the concentration gradient of H+ ions (i.e., protons) across the inner membrane, which is relatively ...
... The theory of chemiosmotic coupling explains how the concentration gradient of H+ is used to generate energy to make ATP. a. The enzyme complex ATP synthase synthesizes ATP using the energy stored in the concentration gradient of H+ ions (i.e., protons) across the inner membrane, which is relatively ...
Chapter 7
... To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please note: once you have used any of the animation functions (such as Play or Pause), you must first click in the white background before you advance the next slide. ...
... To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please note: once you have used any of the animation functions (such as Play or Pause), you must first click in the white background before you advance the next slide. ...
Slide 1
... - strong activator - produced by PFK-2 when excess fru-6-phosphate - indirect means of substrate stimulation or feed forward activation ...
... - strong activator - produced by PFK-2 when excess fru-6-phosphate - indirect means of substrate stimulation or feed forward activation ...
What happened to my cousin Patrick O’Neill?
... Q3: The high energy phosphate bond in ATP is _____ and ____ energy to break the bond. A: Easy to break, releases B: Hard to break, requires C: Easy to break, requires D: Hard to break, releases ...
... Q3: The high energy phosphate bond in ATP is _____ and ____ energy to break the bond. A: Easy to break, releases B: Hard to break, requires C: Easy to break, requires D: Hard to break, releases ...
Bio 101
... substances across the membrane. Uses ATP phosphorylation to activate transport protein » Exocytosis- cellular expulsion of molecules using cellular energy » Endocytosis- cellular intake of macromolecules using cellular energy ─ pinocytosis-cellular intake of fluid droplets ─ phagocytosis- engulfing ...
... substances across the membrane. Uses ATP phosphorylation to activate transport protein » Exocytosis- cellular expulsion of molecules using cellular energy » Endocytosis- cellular intake of macromolecules using cellular energy ─ pinocytosis-cellular intake of fluid droplets ─ phagocytosis- engulfing ...
Citric Acid cycle or Tricarboxylic Acid cycle or Krebs Cycle
... Thiamine pyrophosphate (TPP) is an important cofactor of pyruvate dehydrogenase complex, or PDC a critical enzyme in glucose metabolism. Thiamine is neither synthesized nor stored in good amounts by most vertebrates. It is required in the diets of most vertebrates. Thiamine deficiency ultimate ...
... Thiamine pyrophosphate (TPP) is an important cofactor of pyruvate dehydrogenase complex, or PDC a critical enzyme in glucose metabolism. Thiamine is neither synthesized nor stored in good amounts by most vertebrates. It is required in the diets of most vertebrates. Thiamine deficiency ultimate ...
CELLULAR RESPIRATION
... • Oxygen is final electron acceptor; water is formed • ADP is converted to ATP by adding phosphate ...
... • Oxygen is final electron acceptor; water is formed • ADP is converted to ATP by adding phosphate ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑