Questions 6 Metabolism_1
... b) Oxidative phosphorylation. c) Substrate-level phosphorylation. d) An aldolase catalyzed reaction. e) An electron transport reaction. 7) Oxidative phosphorylation uses ALL of the following for energy production EXCEPT: a) electrons from NADH. b) membrane-associated electron transport chain. c) an ...
... b) Oxidative phosphorylation. c) Substrate-level phosphorylation. d) An aldolase catalyzed reaction. e) An electron transport reaction. 7) Oxidative phosphorylation uses ALL of the following for energy production EXCEPT: a) electrons from NADH. b) membrane-associated electron transport chain. c) an ...
Pthways and metabolites of microbial cells
... to produce beer and wine. Tests to detect fermentation end products are often used in the microbiology lab to identify bacteria and other microbes. Animal muscle cells also undergo fermentation when not enough oxygen is delivered to cells during intense exercise. In this case, the fermentation end p ...
... to produce beer and wine. Tests to detect fermentation end products are often used in the microbiology lab to identify bacteria and other microbes. Animal muscle cells also undergo fermentation when not enough oxygen is delivered to cells during intense exercise. In this case, the fermentation end p ...
ATP Molecules
... the cytoplasm with no oxygen needed; yields 2 ATP Really – 2 ATP 4 ATP (net 2) Occurs without oxygen (anaerobic) In cytoplasm – mitochondria not needed ...
... the cytoplasm with no oxygen needed; yields 2 ATP Really – 2 ATP 4 ATP (net 2) Occurs without oxygen (anaerobic) In cytoplasm – mitochondria not needed ...
Fermentation Fermentation is an ancient mode of metabolism, and it
... an organism's ability to ferment certain sugars, or examining an organisms's array of end products) in order to identify them, down to the genus level. ...
... an organism's ability to ferment certain sugars, or examining an organisms's array of end products) in order to identify them, down to the genus level. ...
Grade 12 Review Answers
... by predators and by temporary periods without food. Classify these species as rselected or K-selected and explain your classifications. Rabbit: r-selected – short life span and quick reproduction (early maturity, lots of babies, lots of reproductive cycles) Tortoise: K-selected – long life span and ...
... by predators and by temporary periods without food. Classify these species as rselected or K-selected and explain your classifications. Rabbit: r-selected – short life span and quick reproduction (early maturity, lots of babies, lots of reproductive cycles) Tortoise: K-selected – long life span and ...
STARVE-FEED CYCLE 1) WELL-FED STATE (food intake
... • ↑ malonyl-CoA inhibits carnitine palmitoyl transferase I (= β-oxidation) 3) covalent modification of enzymes • phosphorylation (protein kinases) / dephosphorylation (protein phosphatases) • some phosphorylated enzymes are active (glycogen phosphorylase) other inactive (glycogen synthase) 4) change ...
... • ↑ malonyl-CoA inhibits carnitine palmitoyl transferase I (= β-oxidation) 3) covalent modification of enzymes • phosphorylation (protein kinases) / dephosphorylation (protein phosphatases) • some phosphorylated enzymes are active (glycogen phosphorylase) other inactive (glycogen synthase) 4) change ...
Cellular respiration - how cells make energy
... - multi carbon compound looses electrons (as two hydrogens). - NAD+ gets the electrons and becomes NADH. It also picks up a hydrogen atom in the process. - another hydrogen atom (ion) is put into the solution surrounding the membrane. - NADH will use its new found energy in an electron transport cha ...
... - multi carbon compound looses electrons (as two hydrogens). - NAD+ gets the electrons and becomes NADH. It also picks up a hydrogen atom in the process. - another hydrogen atom (ion) is put into the solution surrounding the membrane. - NADH will use its new found energy in an electron transport cha ...
Bioenergetics
... Energy investment phase o requires ATP Energy generation phase o produces ATP, “NADH” (carrier molecule), & pyruvic acid or lactic acid Key Points in Glycolysis Define – breakdown of ONLY glucose to make ATP Energy put in to get going (phosphorylation) Enzymes needed throughout ...
... Energy investment phase o requires ATP Energy generation phase o produces ATP, “NADH” (carrier molecule), & pyruvic acid or lactic acid Key Points in Glycolysis Define – breakdown of ONLY glucose to make ATP Energy put in to get going (phosphorylation) Enzymes needed throughout ...
USMLE Step 1 Web Prep — Glycolysis and Pyruvate
... The correct answer is D Galactosemia occurs in two very different clinical forms. Deficiency of galactokinase produces very mild disease with the only significant complication being cataract formation.In contrast, homozygous deficiency of galactose-1-phosphate uridyltransferase produces severe disea ...
... The correct answer is D Galactosemia occurs in two very different clinical forms. Deficiency of galactokinase produces very mild disease with the only significant complication being cataract formation.In contrast, homozygous deficiency of galactose-1-phosphate uridyltransferase produces severe disea ...
Exam 3 Q2 Review Sheet 1/2/11
... why they cause a problem. For example, why would DNP be an excellent weight loss drug? 27. It turns out that you need only very small amounts of vitamin B3 (niacin), which is used to make NAD+. The same goes for riboflavin, the vitamin used in the synthesis of FAD. However, you have incredible numbe ...
... why they cause a problem. For example, why would DNP be an excellent weight loss drug? 27. It turns out that you need only very small amounts of vitamin B3 (niacin), which is used to make NAD+. The same goes for riboflavin, the vitamin used in the synthesis of FAD. However, you have incredible numbe ...
Chapter Outline
... 5. Glucose is a high-energy molecule; CO2 and H2O are low-energy molecules; cellular respiration is thus exergonic because it releases energy. 6. Electrons are removed from substrates and received by oxygen, which combines with H + to become water. 7. Glucose is oxidized and O2 is reduced. 8. The re ...
... 5. Glucose is a high-energy molecule; CO2 and H2O are low-energy molecules; cellular respiration is thus exergonic because it releases energy. 6. Electrons are removed from substrates and received by oxygen, which combines with H + to become water. 7. Glucose is oxidized and O2 is reduced. 8. The re ...
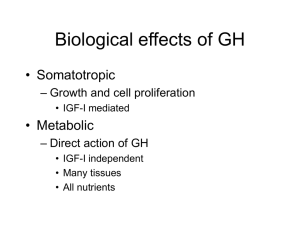
Biological effects of GH
... – Different between adipose and muscle – Growth of muscle in response to GH • Depends on availability of dietary proteins and ...
... – Different between adipose and muscle – Growth of muscle in response to GH • Depends on availability of dietary proteins and ...
Anaerobic and aerobic oxidation of glucose
... transformed to pyruvate with production of a small amount of energy in the form of ATP or NADH. Glycolysis is an anaerobic process (it does not require oxygen). Glycolysis pathway is used by anaerobic as well as aerobic organisms. In glycolysis one molecule of glucose is converted into two molecules ...
... transformed to pyruvate with production of a small amount of energy in the form of ATP or NADH. Glycolysis is an anaerobic process (it does not require oxygen). Glycolysis pathway is used by anaerobic as well as aerobic organisms. In glycolysis one molecule of glucose is converted into two molecules ...
Biochemistry File - Northwest ISD Moodle
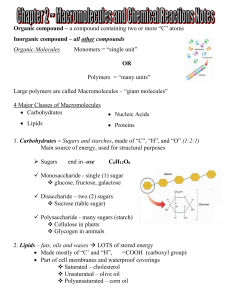
... 4. Proteins – polymers of amino acids joined by peptide bonds Used to build cells, transport molecules, and control the rate of reactions Made of “C”, “H”, “O”, and “N” 20 different amino acids ...
... 4. Proteins – polymers of amino acids joined by peptide bonds Used to build cells, transport molecules, and control the rate of reactions Made of “C”, “H”, “O”, and “N” 20 different amino acids ...
Biological Macromolecules
... **Antibodies are part of the immune system. **When something enters the body that isn’t supposed to be there, like certain bacteria, antibodies find the invader and stick themselves onto it. **White Blood cells destroy the invaders (hopefully) ...
... **Antibodies are part of the immune system. **When something enters the body that isn’t supposed to be there, like certain bacteria, antibodies find the invader and stick themselves onto it. **White Blood cells destroy the invaders (hopefully) ...
Notes - PDST
... This takes place in the cytoplasm. It does not use oxygen. It releases a small amount of energy .At the end of this stage, glucose has been broken down into two molecules of pyruvate. Stage 2 This stage takes place in the mitochondria. This stage uses oxygen. The two molecules of pyruvate go into th ...
... This takes place in the cytoplasm. It does not use oxygen. It releases a small amount of energy .At the end of this stage, glucose has been broken down into two molecules of pyruvate. Stage 2 This stage takes place in the mitochondria. This stage uses oxygen. The two molecules of pyruvate go into th ...
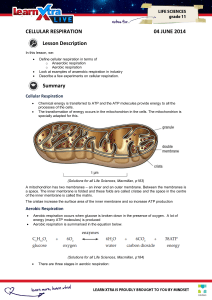
INTRODUCTION TO CELLULAR RESPIRATION
... – At this point, the acetyl group associates with a fourcarbon molecule forming a six-carbon molecule – The six-carbon molecule then passes through a series of redox reactions that regenerate the four-carbon molecule (thus the “cycle” designation) ...
... – At this point, the acetyl group associates with a fourcarbon molecule forming a six-carbon molecule – The six-carbon molecule then passes through a series of redox reactions that regenerate the four-carbon molecule (thus the “cycle” designation) ...
Citric Acid Cycle
... • Three NADH, one FADH2 & 1 GTP/ATP is made in the citric acid cycle. • The citric acid cycle can be used to make precursors for ...
... • Three NADH, one FADH2 & 1 GTP/ATP is made in the citric acid cycle. • The citric acid cycle can be used to make precursors for ...
File
... 1. Amount of sunlight 2. Amount of water 3. Amount of carbon dioxide CHEMOSYNTHESIS - Process by which some organisms produce energy through a chemical reaction. (ex. Bacteria) These are also autotrophs. CELLULAR RESPIRATION The process by which food (glucose) is broken down to produce ATP. Who ...
... 1. Amount of sunlight 2. Amount of water 3. Amount of carbon dioxide CHEMOSYNTHESIS - Process by which some organisms produce energy through a chemical reaction. (ex. Bacteria) These are also autotrophs. CELLULAR RESPIRATION The process by which food (glucose) is broken down to produce ATP. Who ...
Chapter 3—The Cell I. Cell Theory. a. Organisms are made of 1 or
... i. When a molecule or substance gives up one or more electrons, it is said to be "oxidized." ii. When a molecule or substance accepts electrons, it is said to be "reduced." iii. LEO the lion goes GER. 1. Electrons don’t exist in isolation; protons (H+) follow… a. 1 electron + 1 proton = 1 hydrogen a ...
... i. When a molecule or substance gives up one or more electrons, it is said to be "oxidized." ii. When a molecule or substance accepts electrons, it is said to be "reduced." iii. LEO the lion goes GER. 1. Electrons don’t exist in isolation; protons (H+) follow… a. 1 electron + 1 proton = 1 hydrogen a ...
幻灯片 1
... and occupy distinct zones where the environmental conditions favour their specific activities. ...
... and occupy distinct zones where the environmental conditions favour their specific activities. ...
Carbohydrate Metabolism Synopsis of Glycolytic Enzyme Deficiencies
... Note: Normally about 10-20% of red cell glycolytic pathways are shunted toward 2,3 DPG formation. This is an important shunt in the red cells and it is normally stimulated on the tissue sides as a result of accumulation of metabolic byproducts such as CO2 and hydrogen ions that drop the pH. Glucose ...
... Note: Normally about 10-20% of red cell glycolytic pathways are shunted toward 2,3 DPG formation. This is an important shunt in the red cells and it is normally stimulated on the tissue sides as a result of accumulation of metabolic byproducts such as CO2 and hydrogen ions that drop the pH. Glucose ...
AP Biology Discussion Notes Thursday 121516
... kinds of organic molecules into cellular respiration • Glycolysis accepts a wide range of ...
... kinds of organic molecules into cellular respiration • Glycolysis accepts a wide range of ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑