Document
... compound. In these steps some energy is released to form ATP (the only ATP formed in glycolysis), and a hydrogen atom is also released. This hydrogen atom is very important as it stores energy, which is later used by the respiratory chain to make more ATP. The hydrogen atom is taken up and carried t ...
... compound. In these steps some energy is released to form ATP (the only ATP formed in glycolysis), and a hydrogen atom is also released. This hydrogen atom is very important as it stores energy, which is later used by the respiratory chain to make more ATP. The hydrogen atom is taken up and carried t ...
1) Which of the following statements describes the results of this
... 4) In the presence of oxygen, the three-carbon compound pyruvate can be catabolized in the citric acid cycle. First, however, the pyruvate 1) loses a carbon, which is given off as a molecule of CO 2, 2) is oxidized to form a two-carbon compound called acetate, and 3) is bonded to coenzyme A. These t ...
... 4) In the presence of oxygen, the three-carbon compound pyruvate can be catabolized in the citric acid cycle. First, however, the pyruvate 1) loses a carbon, which is given off as a molecule of CO 2, 2) is oxidized to form a two-carbon compound called acetate, and 3) is bonded to coenzyme A. These t ...
Biology 2107/03
... and through the membrane (the “free-wheeling” model). The proteins can move transversely (“flip-flop”) from one side of the membrane to the other, but they cannot move laterally (side-toside) (the “trans-crystalline” model). The proteins can move laterally (side-to-side), but they cannot transversel ...
... and through the membrane (the “free-wheeling” model). The proteins can move transversely (“flip-flop”) from one side of the membrane to the other, but they cannot move laterally (side-toside) (the “trans-crystalline” model). The proteins can move laterally (side-to-side), but they cannot transversel ...
THE SCIENTIFIC METHOD Define problem Research and collect
... Catalase catalyzes the breakdown of hydrogen peroxide to water and oxygen. The name of an enzyme is formed by replacing the usual ending of a substrate’s name with the ending “ase.” Amylase – catalyze the reactions of the carbohydrates (Ptyalin, enzyme found in saliva that begins chemical breakdown ...
... Catalase catalyzes the breakdown of hydrogen peroxide to water and oxygen. The name of an enzyme is formed by replacing the usual ending of a substrate’s name with the ending “ase.” Amylase – catalyze the reactions of the carbohydrates (Ptyalin, enzyme found in saliva that begins chemical breakdown ...
Cellular Metabolism
... The entire process of synthesizing ATP in the electron transport system is called oxidative phosphorylation. The ATP is produced by chemiosmosis. 2. Chemiosmotic Theory of ATP Production – Chemi – chemical forces, Osmosis – pushing forces The chemiosmotic theory of ATP production is based on the fac ...
... The entire process of synthesizing ATP in the electron transport system is called oxidative phosphorylation. The ATP is produced by chemiosmosis. 2. Chemiosmotic Theory of ATP Production – Chemi – chemical forces, Osmosis – pushing forces The chemiosmotic theory of ATP production is based on the fac ...
Fatty Acid Catabolism
... listed below. Give the proper reaction types in the order that they occur in the β-oxidation pathway. ...
... listed below. Give the proper reaction types in the order that they occur in the β-oxidation pathway. ...
6 Energy and Metabolism
... the mitochondria. Here, the NADH molecules from glycolysis and the TCA cycle are oxidized back to NAD so glycolysis can continue. It also generates 3 more ATP. When this system is performing in the presence of oxygen, oxygen is consumed and the waste product is water. When it is done anaerobically ( ...
... the mitochondria. Here, the NADH molecules from glycolysis and the TCA cycle are oxidized back to NAD so glycolysis can continue. It also generates 3 more ATP. When this system is performing in the presence of oxygen, oxygen is consumed and the waste product is water. When it is done anaerobically ( ...
CHEMICAL REACTIONS, ENZYMES, ATP, CELLULAR
... 7. Where in the cell does the electron transport chain part of cellular respiration occur? 8. Is ETC aerobic or anaerobic? 9. How many ATP (net) are made in the glycolysis part of cellular respiration? ...
... 7. Where in the cell does the electron transport chain part of cellular respiration occur? 8. Is ETC aerobic or anaerobic? 9. How many ATP (net) are made in the glycolysis part of cellular respiration? ...
Electron Transport Chain - Dr-Manar-KSU
... 4-the net yield of ATP by substrate level phosphorylation during citric acid cycle for each glucose molecule is two. 5- FADH2 is an electron carrier in glycolysis. ...
... 4-the net yield of ATP by substrate level phosphorylation during citric acid cycle for each glucose molecule is two. 5- FADH2 is an electron carrier in glycolysis. ...
Fibrous proteins are especially abundant outside the cell, where
... sugars by photosynthesis, whereas animals obtain sugars by eating other organisms. If a fuel molecule such as glucose were oxidized to CO2 and H2O in a single step (as happens in nonliving systems), it would release an amount of energy many times larger than any carrier molecule could capture. Inste ...
... sugars by photosynthesis, whereas animals obtain sugars by eating other organisms. If a fuel molecule such as glucose were oxidized to CO2 and H2O in a single step (as happens in nonliving systems), it would release an amount of energy many times larger than any carrier molecule could capture. Inste ...
BIOL 303 Cell Biology Test preparation questionnaire # 1
... 1. How does the sign and magnitude of the ∆G for a reaction indicate whether it can occur spontaneously? 55. By what means can cells carry out chemical transformations that are thrmodynamically unfavorable? 56. Can enzymes change the ∆G for a reaction and thus make an unfavorable reaction happen? 57 ...
... 1. How does the sign and magnitude of the ∆G for a reaction indicate whether it can occur spontaneously? 55. By what means can cells carry out chemical transformations that are thrmodynamically unfavorable? 56. Can enzymes change the ∆G for a reaction and thus make an unfavorable reaction happen? 57 ...
Block 1 Unit 2 Objectives Bone Tissue Objectives List and describe
... 1. The three types of muscle tissue are Skeletal, Cardiac, and Smooth. Skeletal muscle causes movement/stature of the body. They act on bone tissue to exert a force in a direction. They are striated muscle tissue in that the myofibrils are organized and inline with one another. They are multinuclea ...
... 1. The three types of muscle tissue are Skeletal, Cardiac, and Smooth. Skeletal muscle causes movement/stature of the body. They act on bone tissue to exert a force in a direction. They are striated muscle tissue in that the myofibrils are organized and inline with one another. They are multinuclea ...
METABOLISM
... and contributes to steady-state equilibria of a stable internal environment. 7. Independent regulation of opposite metabolic pathways is facilitated by their localization in different cell compartments (cytoplasm, organelles), cells or organs. ...
... and contributes to steady-state equilibria of a stable internal environment. 7. Independent regulation of opposite metabolic pathways is facilitated by their localization in different cell compartments (cytoplasm, organelles), cells or organs. ...
Slide 1
... undergoes some chemical grooming in which – a carboxyl group is removed and given off as CO2, – the two-carbon compound remaining is oxidized while a molecule of NAD+ is reduced to NADH, – coenzyme A joins with the two-carbon group to form acetyl coenzyme A, abbreviated as acetyl CoA, and – acetyl C ...
... undergoes some chemical grooming in which – a carboxyl group is removed and given off as CO2, – the two-carbon compound remaining is oxidized while a molecule of NAD+ is reduced to NADH, – coenzyme A joins with the two-carbon group to form acetyl coenzyme A, abbreviated as acetyl CoA, and – acetyl C ...
Week 4 met 2 kin 310
... 1. Describe the activation and translocation of free fatty acids into skeletal muscle that is required prior to metabolism as fuel. (do not include the regulation of translocation in your answer). 2. Describe the mobilization, circulation and uptake of free fatty acids during exercise. Why do resear ...
... 1. Describe the activation and translocation of free fatty acids into skeletal muscle that is required prior to metabolism as fuel. (do not include the regulation of translocation in your answer). 2. Describe the mobilization, circulation and uptake of free fatty acids during exercise. Why do resear ...
Biology First Semester Study Questions
... 11. DNA, RNA 12. DNA= heredity codes; RNA= protein synthesis 13. both 14. animal structures, enzymes, stores nutrients, defend against disease 15. both 16. speed up chemical reactions by lowering activation energy 17. Denaturation means an enzyme changes shape, making it useless. Two causes are heat ...
... 11. DNA, RNA 12. DNA= heredity codes; RNA= protein synthesis 13. both 14. animal structures, enzymes, stores nutrients, defend against disease 15. both 16. speed up chemical reactions by lowering activation energy 17. Denaturation means an enzyme changes shape, making it useless. Two causes are heat ...
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + Energy (ATP)
... Energy investment phase: Steps 1 – 4 How many ATP's required (used)? Results in 2 molecules of 3 phosphoglyceraldehyde (G3P) ...
... Energy investment phase: Steps 1 – 4 How many ATP's required (used)? Results in 2 molecules of 3 phosphoglyceraldehyde (G3P) ...
chapter_14_respiration_in_plants
... ETS or electron transport system is located in the inner mitochondrial membrane. It helps in releasing and utilizing the energy stored in NADH+H+ and FADH2. NADH + H+, which is formed during glycolysis and citric acid cycle, gets oxidized by NADH dehydrogenase (complex I). The electrons so generated ...
... ETS or electron transport system is located in the inner mitochondrial membrane. It helps in releasing and utilizing the energy stored in NADH+H+ and FADH2. NADH + H+, which is formed during glycolysis and citric acid cycle, gets oxidized by NADH dehydrogenase (complex I). The electrons so generated ...
Electron Transport Chain _ETC
... Energy-rich molecules, such as glucose, are metabolized by a series of oxidation reactions ultimately yielding Co2 and water. The metabolic intermediates of these reactions donate electrons to specific coenzymes ( NAD+,FAD) and The reduced form of these coenzymes ( NADH,FADH2) can, in turn, each don ...
... Energy-rich molecules, such as glucose, are metabolized by a series of oxidation reactions ultimately yielding Co2 and water. The metabolic intermediates of these reactions donate electrons to specific coenzymes ( NAD+,FAD) and The reduced form of these coenzymes ( NADH,FADH2) can, in turn, each don ...
Cellular Respiration
... H+ ions across a membrane – The energy of the gradient is harnessed to make ATP by the process of chemiosmosis ...
... H+ ions across a membrane – The energy of the gradient is harnessed to make ATP by the process of chemiosmosis ...
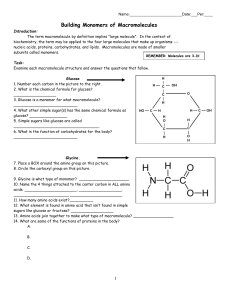
Building Monomers of Macromolecules
... Building Monomers of Macromolecules Introduction: The term macromolecule by definition implies "large molecule". In the context of biochemistry, the term may be applied to the four large molecules that make up organisms --nucleic acids, proteins, carbohydrates, and lipids. Macromolecules are made of ...
... Building Monomers of Macromolecules Introduction: The term macromolecule by definition implies "large molecule". In the context of biochemistry, the term may be applied to the four large molecules that make up organisms --nucleic acids, proteins, carbohydrates, and lipids. Macromolecules are made of ...
First Semester Biology Exam
... 66. Adenosine Triphosphate, what the body uses for energy, the last phosphate bond stores the most energy, it is a specialized nucleotide, becomes ADP when bonds are broken, necessary for cells to do tasks. Sugar is ribose, the base is Adenine. 67. oxygen 68. release stored energy and converts that ...
... 66. Adenosine Triphosphate, what the body uses for energy, the last phosphate bond stores the most energy, it is a specialized nucleotide, becomes ADP when bonds are broken, necessary for cells to do tasks. Sugar is ribose, the base is Adenine. 67. oxygen 68. release stored energy and converts that ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑