supporting information
... Correspondence concerning this article should be addressed to Raymond Zeng at ...
... Correspondence concerning this article should be addressed to Raymond Zeng at ...
dehydration synthesis
... ATP; when energy is released, ATP becomes ADP, ready to be regenerated into ATP. ...
... ATP; when energy is released, ATP becomes ADP, ready to be regenerated into ATP. ...
Hardy-Weinberg Assignment
... membrane; these protons then flow through ATP synthase which converts ADP to ATP ...
... membrane; these protons then flow through ATP synthase which converts ADP to ATP ...
Lecture-Intro to metabolism - Creighton Chemistry Webserver
... Rxns extremely efficient, no accumulating intermediates Stereospecific rxns are the rule Everything occurs under constant conditions ...
... Rxns extremely efficient, no accumulating intermediates Stereospecific rxns are the rule Everything occurs under constant conditions ...
Key Terms PDF - QuizOver.com
... transfer of an amine group from one molecule to another as a way to turn nitrogen waste into ammonia ...
... transfer of an amine group from one molecule to another as a way to turn nitrogen waste into ammonia ...
Cellular Respiration
... Define cellular respiration and state its importance as a life process. Differentiate between aerobic respiration, anaerobic respiration, and fermentation. State and explain the chemical equation for cellular respiration. Define oxidation and reduction and explain the idea of redox reactions. Explai ...
... Define cellular respiration and state its importance as a life process. Differentiate between aerobic respiration, anaerobic respiration, and fermentation. State and explain the chemical equation for cellular respiration. Define oxidation and reduction and explain the idea of redox reactions. Explai ...
Karbohidrat Metabolizması
... Inhibitors of carnitine palmitoyl transferase 1, especially cyclo-oxirane derivatives which are activated by fatty-acyl CoA synthetase to their CoA derivative which inhibits CPT1 with Ki values of less than 1mM. ...
... Inhibitors of carnitine palmitoyl transferase 1, especially cyclo-oxirane derivatives which are activated by fatty-acyl CoA synthetase to their CoA derivative which inhibits CPT1 with Ki values of less than 1mM. ...
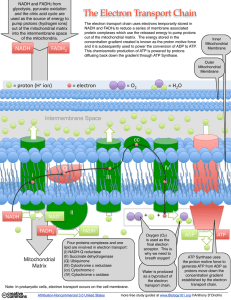
What is the Electron Transport Chain?
... NADH and FADH2 from glycolysis, pyruvate oxidation and the citric acid cycle are used as the source of energy to pump protons (hydrogen ions) out of the mitochondrial matrix into the intermembrane space of the mitochondria. ...
... NADH and FADH2 from glycolysis, pyruvate oxidation and the citric acid cycle are used as the source of energy to pump protons (hydrogen ions) out of the mitochondrial matrix into the intermembrane space of the mitochondria. ...
Chapter 9: Glycolysis & Krebs Cycle
... reactions in living cells in which sugars are broken down and energy is released Mitochondria in ...
... reactions in living cells in which sugars are broken down and energy is released Mitochondria in ...
Biochemistry (Unit 1) Exam Review
... structure and briefly describe the structures of globular and fibrous proteins. ANSWER: Primary structure is the number and sequence of amino acids in a polypeptide strand. These polypeptides usually consist of between 50 to 100 amino acids. The primary structure is determined by the nucleotide sequ ...
... structure and briefly describe the structures of globular and fibrous proteins. ANSWER: Primary structure is the number and sequence of amino acids in a polypeptide strand. These polypeptides usually consist of between 50 to 100 amino acids. The primary structure is determined by the nucleotide sequ ...
Exam #3 Review Exam #3 will cover from glycolysis to complex
... for phosphorylation of ADP to form ATP! ATP synthase - allows protons pumped out during production of the PMF to pass back into the cell ---> uses energy to fuel the phosphorylation of ADP to produce ATP. This is oxidative phosphorylation! • Practice: If 5 molecules of NADH are completely oxidized b ...
... for phosphorylation of ADP to form ATP! ATP synthase - allows protons pumped out during production of the PMF to pass back into the cell ---> uses energy to fuel the phosphorylation of ADP to produce ATP. This is oxidative phosphorylation! • Practice: If 5 molecules of NADH are completely oxidized b ...
Bio 216 Exam 1 Name Date 1. The study of how disease or injury
... 26. Nervous tissue is specialized to produce and conduct electrical impulses. A. True B. False 27. One exocrine function of the skin is the synthesis and secretion of melanin from the sebaceous glands. A. True B. False 28. Enzymes ______________ the rate of a specific chemical reaction. A. decrease ...
... 26. Nervous tissue is specialized to produce and conduct electrical impulses. A. True B. False 27. One exocrine function of the skin is the synthesis and secretion of melanin from the sebaceous glands. A. True B. False 28. Enzymes ______________ the rate of a specific chemical reaction. A. decrease ...
Fermentation Due: April 19th by 5:00 PM Please submit your
... 3. Why is anaerobic respiration necessary to make beer and wine? Under aerobic conditions, pyruvate (the product of glycolysis) will preferentially transition into the TCA cycle to make energy. However, in the absence of O2, the last step in the aerobic respiration cannot happen. Consequently, the p ...
... 3. Why is anaerobic respiration necessary to make beer and wine? Under aerobic conditions, pyruvate (the product of glycolysis) will preferentially transition into the TCA cycle to make energy. However, in the absence of O2, the last step in the aerobic respiration cannot happen. Consequently, the p ...
Document
... Carbohydrates – 4 kcal/gm Proteins – 4 kcal/gm Fats – 9 kcal/gm Calorie = heat required to raise the temperature of water by 10c Kcal = 1000cal Cell Respiration = process that “burns” food Carbs = quick energy release Fats, proteins = slow to release energy ...
... Carbohydrates – 4 kcal/gm Proteins – 4 kcal/gm Fats – 9 kcal/gm Calorie = heat required to raise the temperature of water by 10c Kcal = 1000cal Cell Respiration = process that “burns” food Carbs = quick energy release Fats, proteins = slow to release energy ...
CO 2 - cloudfront.net
... energy in the form of ATP BUT don’t forget its also the ways we produce all our heat. • Also don’t forget we can use other sugars, fats and amino acids to produce energy in the form of ATP using these same pathways. • The two major linked mechanisms for achieving this are Glycolysis and Krebs Cycle ...
... energy in the form of ATP BUT don’t forget its also the ways we produce all our heat. • Also don’t forget we can use other sugars, fats and amino acids to produce energy in the form of ATP using these same pathways. • The two major linked mechanisms for achieving this are Glycolysis and Krebs Cycle ...
Alcohol Metabolism - Jessica Leary Nutrition Portfolio
... What is Alcohol? Ethyl alcohol, or ethanol, is the common alcohol that will make one intoxicated when ingested. This is the chemical this is found in beer, wine, and liquor. ...
... What is Alcohol? Ethyl alcohol, or ethanol, is the common alcohol that will make one intoxicated when ingested. This is the chemical this is found in beer, wine, and liquor. ...
Where is DNA in a euk cell?
... A. primary B. secondary C. tertiary D. quaternary Microtubules and Microfilaments What do they have in common? A. components of the cytoskeleton B. made of tubulin C. only found in plant cells D. only found in bacterial cells Breaking down proteins into amino acids a. hydrolysis b. condensation The ...
... A. primary B. secondary C. tertiary D. quaternary Microtubules and Microfilaments What do they have in common? A. components of the cytoskeleton B. made of tubulin C. only found in plant cells D. only found in bacterial cells Breaking down proteins into amino acids a. hydrolysis b. condensation The ...
Microbial Metabolism
... electrons are higher (more negative) on the tower • To determine which direction the reactions go, see which is “higher” on the electron tower • Note the position of important electron carriers (NAD, FAD, cytochrome a) and external electron donors/acceptors (H2, organic compounds, O2) ...
... electrons are higher (more negative) on the tower • To determine which direction the reactions go, see which is “higher” on the electron tower • Note the position of important electron carriers (NAD, FAD, cytochrome a) and external electron donors/acceptors (H2, organic compounds, O2) ...
21. Which of the electron carriers in the electron transport
... non-amino acid components? a) cytochrome c b) FMN c) *ubiquinone d) none of the above e) all of the above 24. An uncoupling protein would do the following a) transport protons against a concentration gradient b) function as a source of heat production c) stop ATP synthesis d) decrease the rate of el ...
... non-amino acid components? a) cytochrome c b) FMN c) *ubiquinone d) none of the above e) all of the above 24. An uncoupling protein would do the following a) transport protons against a concentration gradient b) function as a source of heat production c) stop ATP synthesis d) decrease the rate of el ...
Slide 1
... • oxidative phosphorylation • Oxidizes pyruvate to ATP & CO2 • Text pg 117 • So why is ATP so important? ...
... • oxidative phosphorylation • Oxidizes pyruvate to ATP & CO2 • Text pg 117 • So why is ATP so important? ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑