Practice Test for BIO 311C
... about an oxidation-reduction (or redox) reaction? A) The molecule that is oxidized loses electrons. B) The molecule that is reduced gains electrons. C) The molecule that is reduced loses electrons. D) The molecule that is oxidized gains electrons. E) Both A and B are correct. 53) Which of the follow ...
... about an oxidation-reduction (or redox) reaction? A) The molecule that is oxidized loses electrons. B) The molecule that is reduced gains electrons. C) The molecule that is reduced loses electrons. D) The molecule that is oxidized gains electrons. E) Both A and B are correct. 53) Which of the follow ...
2.2.5-H.2.2.10 Respiration - Intermediate School Biology
... Respiration is a two stage process: The first stage does not require oxygen and releases a small amount of energy; The second stage does require oxygen and releases a large amount of energy. Note: Further detail on stages 1 and 2 under higher level P 3 First stage process Anaerobic respiration ...
... Respiration is a two stage process: The first stage does not require oxygen and releases a small amount of energy; The second stage does require oxygen and releases a large amount of energy. Note: Further detail on stages 1 and 2 under higher level P 3 First stage process Anaerobic respiration ...
Cellular Respiration and Fermentation
... • Obligate anaerobes carry out fermentation or anaerobic respiration and cannot survive in the presence of O2 • Yeast and many bacteria are facultative anaerobes, meaning that they can survive using either fermentation or cellular respiration • In a facultative anaerobe, pyruvate is a fork in the m ...
... • Obligate anaerobes carry out fermentation or anaerobic respiration and cannot survive in the presence of O2 • Yeast and many bacteria are facultative anaerobes, meaning that they can survive using either fermentation or cellular respiration • In a facultative anaerobe, pyruvate is a fork in the m ...
LessonPlansInc.com
... 1st Review with students about the purpose of cellular respiration, the active transport with hydrogen pumps, ATP synthase, and how NADH and FADH2 are electron carriers for the electron transport chain. 2nd Demonstrate to the students the activity. Ask for one student volunteer. Have that student be ...
... 1st Review with students about the purpose of cellular respiration, the active transport with hydrogen pumps, ATP synthase, and how NADH and FADH2 are electron carriers for the electron transport chain. 2nd Demonstrate to the students the activity. Ask for one student volunteer. Have that student be ...
Biochemistry I, Spring Term 2001 - Third Exam:
... C2. (15 pts) Answer ONE of the following three questions. i) In biosynthetic and degradative pathways, several steps are similar, often catalyzed by the same enzyme. Other steps are different, catalyzed by one or more different enzymes. As an example of the latter, pick one such step in either glyco ...
... C2. (15 pts) Answer ONE of the following three questions. i) In biosynthetic and degradative pathways, several steps are similar, often catalyzed by the same enzyme. Other steps are different, catalyzed by one or more different enzymes. As an example of the latter, pick one such step in either glyco ...
WHY DO CARDIOMYOCYTES (HEART MUSCLE CELLS) STORE
... anaerobic energy production is extremely limited? A cardiomyocyte, deprived of its blood supply, cannot continue beating for more than a few minutes at the very most. The glycogen would therefore only be ...
... anaerobic energy production is extremely limited? A cardiomyocyte, deprived of its blood supply, cannot continue beating for more than a few minutes at the very most. The glycogen would therefore only be ...
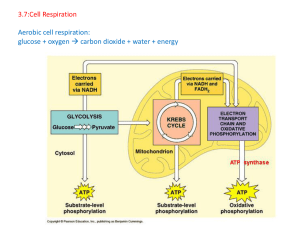
3.7:Cell Respiration Aerobic cell respiration: glucose
... rate can be measured by the disappearance of raw materials / 2 CO (in solution); rate of change of 2 CO can be measured (indirectly) by pH change; rate can be measured by the appearance of products/ 2 O /starch; rate can be measured by measuring rate of change of biomass; description of apparatus to ...
... rate can be measured by the disappearance of raw materials / 2 CO (in solution); rate of change of 2 CO can be measured (indirectly) by pH change; rate can be measured by the appearance of products/ 2 O /starch; rate can be measured by measuring rate of change of biomass; description of apparatus to ...
Gluconeogenesis
... The effects of cortisol on carbohydrate and protein metabolism in the liver. ...
... The effects of cortisol on carbohydrate and protein metabolism in the liver. ...
Prescott`s Microbiology, 9th Edition 12 Anabolism: The Use of
... Triacylglycerols are formed from the reduction of dihydroxyacetone phosphate (a glycolytic pathway intermediate) to glycerol 3-phosphate, which then undergoes esterification with two fatty acids to form phosphatidic acid; this can then be used to produce triacylglycerol Phospholipids also are produc ...
... Triacylglycerols are formed from the reduction of dihydroxyacetone phosphate (a glycolytic pathway intermediate) to glycerol 3-phosphate, which then undergoes esterification with two fatty acids to form phosphatidic acid; this can then be used to produce triacylglycerol Phospholipids also are produc ...
Chapter 4 - Cellular Metabolism
... Oxygen is needed for aerobic respiration, which occurs within the mitochondria. b. There is a much greater gain of ATP molecules from aerobic respiration. c. The final products of glucose oxidation are carbon dioxide, water, and energy. 4.5 Metabolic Pathways (Figs. 4.8, 4.9) A. The enzymes controll ...
... Oxygen is needed for aerobic respiration, which occurs within the mitochondria. b. There is a much greater gain of ATP molecules from aerobic respiration. c. The final products of glucose oxidation are carbon dioxide, water, and energy. 4.5 Metabolic Pathways (Figs. 4.8, 4.9) A. The enzymes controll ...
L10v02b_-_citric_acid_cycle.stamped_doc
... [00:00:00.69] SPEAKER 1: Hi there. In this video clip, we're going to continue our discussion on central metabolism. And we left off with acetyl CoA being produced from either sugar or fatty acids being present in the inner mitochondrial matrix and being just about ready to integrate into the citric ...
... [00:00:00.69] SPEAKER 1: Hi there. In this video clip, we're going to continue our discussion on central metabolism. And we left off with acetyl CoA being produced from either sugar or fatty acids being present in the inner mitochondrial matrix and being just about ready to integrate into the citric ...
Clues from cell metabolism
... acetyl-CoA, ribose and glucose-derived nonessential amino acids. To maintain this high rate of glycolysis, a ready supply of the phosphate acceptor ADP is needed. If cellular metabolism is too efficient, all the ADP will be phosphorylated to ATP, and further glucose metabolism will be inhibited. Can ...
... acetyl-CoA, ribose and glucose-derived nonessential amino acids. To maintain this high rate of glycolysis, a ready supply of the phosphate acceptor ADP is needed. If cellular metabolism is too efficient, all the ADP will be phosphorylated to ATP, and further glucose metabolism will be inhibited. Can ...
Islamic University of Gaza Advanced Biochemistry Faculty of
... C. The citrate synthase reaction is strongly exergonic. Why is this essential to maintaining flux through the TCA cycle? (2 points) Answer: The first reaction of the cycle is condensation of the methyl carbon of acetyl-CoA with the keto carbon (C-2) of oxaloacetate. The standard free energy of the r ...
... C. The citrate synthase reaction is strongly exergonic. Why is this essential to maintaining flux through the TCA cycle? (2 points) Answer: The first reaction of the cycle is condensation of the methyl carbon of acetyl-CoA with the keto carbon (C-2) of oxaloacetate. The standard free energy of the r ...
Lecture 28, Apr 7
... a higher concentration of H+ on this side of the membrane. The resulting difference in pH and electric charge across the membrane is a form of stored energy. The only path available for protons to travel back across the membrane to neutralize the pH and electric charge on both sides of the membrane ...
... a higher concentration of H+ on this side of the membrane. The resulting difference in pH and electric charge across the membrane is a form of stored energy. The only path available for protons to travel back across the membrane to neutralize the pH and electric charge on both sides of the membrane ...
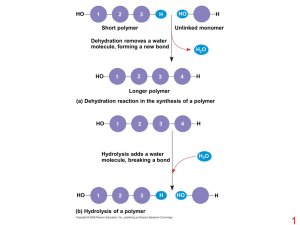
Chapter 6, Section 3
... Organic: contains carbon ◦ All living things contain carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P) Monomer: created when C,H,O, N, P bond together to form small molecules Polymer: large compounds that are formed by joining monomers together ...
... Organic: contains carbon ◦ All living things contain carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P) Monomer: created when C,H,O, N, P bond together to form small molecules Polymer: large compounds that are formed by joining monomers together ...
The Aerobic Fate of Pyruvate
... I could tell that some of you were not impressed by the mere 2 ATPs produced per glucose by glycolysis. The 2 ATP’s produced are only a small fraction of the potential energy available from glucose. Under anaerobic conditions, animals convert glucose into 2 molecules of lactate. Much of the potentia ...
... I could tell that some of you were not impressed by the mere 2 ATPs produced per glucose by glycolysis. The 2 ATP’s produced are only a small fraction of the potential energy available from glucose. Under anaerobic conditions, animals convert glucose into 2 molecules of lactate. Much of the potentia ...
Communication
... in oxidation reactions. This is catalyzed by dehydrogenase enzymes Co enzymes are required to activate the oxidation reactions in respiration Hydrogen atoms becomes attached to ...
... in oxidation reactions. This is catalyzed by dehydrogenase enzymes Co enzymes are required to activate the oxidation reactions in respiration Hydrogen atoms becomes attached to ...
Metabolism
... reactions that provide energy for the production of ATP • This energy is used to generate ATP from phosphorylation of ADP. • It is a series of Redox reactions. ...
... reactions that provide energy for the production of ATP • This energy is used to generate ATP from phosphorylation of ADP. • It is a series of Redox reactions. ...
Nutrition
... Starch is a carbohydrate stored by plants Cellulose = many glucose molecules joined together (plants) Function: Important structural function in plants - it is a component of plant cell walls. Glycogen = many glucose molecules Function: stored by animals (many in liver and muscles) and can be used f ...
... Starch is a carbohydrate stored by plants Cellulose = many glucose molecules joined together (plants) Function: Important structural function in plants - it is a component of plant cell walls. Glycogen = many glucose molecules Function: stored by animals (many in liver and muscles) and can be used f ...
Tricarboxylic acid cycle
... 1. Citrate synthase: inhibited by ATP, NADH, acyl CoA and succinyl CoA 2. Isocitrate dehydrogenase: Inhibited by ATP and NADH and activated by ADP 3. -KG dehydrogenase inhibited by NADH & succinyl CoA The availability of ADP: Important for proceeding the TCA cycle if not oxidation of NADH and FADH2 ...
... 1. Citrate synthase: inhibited by ATP, NADH, acyl CoA and succinyl CoA 2. Isocitrate dehydrogenase: Inhibited by ATP and NADH and activated by ADP 3. -KG dehydrogenase inhibited by NADH & succinyl CoA The availability of ADP: Important for proceeding the TCA cycle if not oxidation of NADH and FADH2 ...
Chapter_02_4E - Ironbark (xtelco)
... is used to resynthesize ATP, preventing the ATP level from decreasing. At exhaustion, both ATP and PCr concentrations are low. ...
... is used to resynthesize ATP, preventing the ATP level from decreasing. At exhaustion, both ATP and PCr concentrations are low. ...
glucose
... • Glucose-1-phosphate can be converted to glucose-6phosphate, which can enter glycolysis • Phosphorylated glucose can’t be absorbed into cells - in the liver and kidneys, glucose-6-phosphate can be hydrolized to glucose • Glycogenolysis is activated by glucogon in the liver and epinephrine in muscle ...
... • Glucose-1-phosphate can be converted to glucose-6phosphate, which can enter glycolysis • Phosphorylated glucose can’t be absorbed into cells - in the liver and kidneys, glucose-6-phosphate can be hydrolized to glucose • Glycogenolysis is activated by glucogon in the liver and epinephrine in muscle ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑