Lecture 15 (Parker) - Department of Chemistry ::: CALTECH
... The citric acid cycle itself does not generate a large amount of ATP, instead it removes electrons from Acetyl CoA forming NADH and FADH2. These electron carriers yield nine ATP molecules when oxidized by oxidative phosphorylation. Electrons released in the re-oxidation of NADH and FADH2 flow throu ...
... The citric acid cycle itself does not generate a large amount of ATP, instead it removes electrons from Acetyl CoA forming NADH and FADH2. These electron carriers yield nine ATP molecules when oxidized by oxidative phosphorylation. Electrons released in the re-oxidation of NADH and FADH2 flow throu ...
Sport`s Nutrition Slides
... the primary pathway used in short-duration maximal efforts, such as power lifting, kicking a soccer ball, or throwing a shotput. This system uses the very small amount of ATP stored in the body and does not require oxygen. This is not much fuel, so to go beyond the ten seconds you will need some oth ...
... the primary pathway used in short-duration maximal efforts, such as power lifting, kicking a soccer ball, or throwing a shotput. This system uses the very small amount of ATP stored in the body and does not require oxygen. This is not much fuel, so to go beyond the ten seconds you will need some oth ...
Answers set 7
... NADPH is not a substrate for oxidative phosphorylation, so deriving its energy equivalence in units of ATP is not straightforward. However the process shown here is one example of several that suggests that in terms of energy equivalence, NADPH = NADH + ATP. The extra energy content of NADPH is the ...
... NADPH is not a substrate for oxidative phosphorylation, so deriving its energy equivalence in units of ATP is not straightforward. However the process shown here is one example of several that suggests that in terms of energy equivalence, NADPH = NADH + ATP. The extra energy content of NADPH is the ...
File
... energy rich. How much more energy do fats carry per gram than carbs? You will have to look this on up 26. Compare trans unsaturated fats to unsaturated fats and explain why one is more healthy than the other. ...
... energy rich. How much more energy do fats carry per gram than carbs? You will have to look this on up 26. Compare trans unsaturated fats to unsaturated fats and explain why one is more healthy than the other. ...
lecture1
... Metabolic pathways can be linear, e.g. glycolysis or can be cyclic, e.g. TCA. In general, the rate of catabolism is controlled not by the conc. of nutrients available in the environment of the cell, but by the cell’s need for energy in the form of ATP. Similarly, the rate of biosynthesis of cell com ...
... Metabolic pathways can be linear, e.g. glycolysis or can be cyclic, e.g. TCA. In general, the rate of catabolism is controlled not by the conc. of nutrients available in the environment of the cell, but by the cell’s need for energy in the form of ATP. Similarly, the rate of biosynthesis of cell com ...
Review for Final Summer 2010
... o Electron transport chain o Fermentation Glycolysis splits sugar to make ATP & NADH Pyruvate from Glycolysis either enter the mitochondria (cellular respiration) or stays in cytosol (one of the two types of fermentation) Fermentation: Alcohol vs. lactic acid (know the difference) Why would ...
... o Electron transport chain o Fermentation Glycolysis splits sugar to make ATP & NADH Pyruvate from Glycolysis either enter the mitochondria (cellular respiration) or stays in cytosol (one of the two types of fermentation) Fermentation: Alcohol vs. lactic acid (know the difference) Why would ...
the Overview - The United Mitochondrial Disease
... As foodstuffs pass down the alimentary canal they are reacted on by sets of enzymes that break down carbohydrates to glucose, fats to fatty acids, and proteins to amino acids, which then circulate in the blood stream. Entry of these nutrients into cells in various tissues is under control of severa ...
... As foodstuffs pass down the alimentary canal they are reacted on by sets of enzymes that break down carbohydrates to glucose, fats to fatty acids, and proteins to amino acids, which then circulate in the blood stream. Entry of these nutrients into cells in various tissues is under control of severa ...
Chp5B - OoCities
... Differ from fat in that the third carbon of glycerol is joined to a negatively charged phosphate group. Hydrocarbon tails are hydrophobic. Polar head (glycerol/phosphate) is hydrophilic. Cluster in water as their hydrophobic tails turn away from water (micelle). Major constituents of cell membranes. ...
... Differ from fat in that the third carbon of glycerol is joined to a negatively charged phosphate group. Hydrocarbon tails are hydrophobic. Polar head (glycerol/phosphate) is hydrophilic. Cluster in water as their hydrophobic tails turn away from water (micelle). Major constituents of cell membranes. ...
Study Guide A - The Science of Payne
... 4. What is the function of the Krebs cycle? a. To produce carbon-based molecules by cellular respiration. b. To produce carbon-based molecules by glycolysis. c. To produce energy-carriers from the breakdown of carbon-based molecules. d. To produce energy-carriers from the synthesis of carbon-based m ...
... 4. What is the function of the Krebs cycle? a. To produce carbon-based molecules by cellular respiration. b. To produce carbon-based molecules by glycolysis. c. To produce energy-carriers from the breakdown of carbon-based molecules. d. To produce energy-carriers from the synthesis of carbon-based m ...
Gokul Das, Ph.D. - Roswell Park Cancer Institute
... Phosphoglycerate dehydrogenase is overexpressed in some cancers and catalyzes a growth-promoting metabolic pathway. Glycolytic cancer cells convert glucose into pyruvate, which can then be oxidized in the mitochondria or converted into lactate. Cells containing enhanced expression of the enzyme phos ...
... Phosphoglycerate dehydrogenase is overexpressed in some cancers and catalyzes a growth-promoting metabolic pathway. Glycolytic cancer cells convert glucose into pyruvate, which can then be oxidized in the mitochondria or converted into lactate. Cells containing enhanced expression of the enzyme phos ...
BOOK NOTES ch9_sec3
... • The cells of most organisms transfer energy found in organic compounds, such as those in foods, to ATP. • The primary fuel for cellular respiration is glucose. Fats can be broken down to make ATP. • Proteins and nucleic acids can also be used to make ATP, but they are usually used for building imp ...
... • The cells of most organisms transfer energy found in organic compounds, such as those in foods, to ATP. • The primary fuel for cellular respiration is glucose. Fats can be broken down to make ATP. • Proteins and nucleic acids can also be used to make ATP, but they are usually used for building imp ...
AP Bio Fall Final Study Guide
... ATP- Adenosine Triphosphate it the form of energy used in our body. ADP- Adenosine Diphosphate the lack of the phosphate group removes one election making it contain no useable energy NADH- Think of these as buses (Thanks Mrs. Groch). They carry electrons on them to the ETC. FADH2- They are a differ ...
... ATP- Adenosine Triphosphate it the form of energy used in our body. ADP- Adenosine Diphosphate the lack of the phosphate group removes one election making it contain no useable energy NADH- Think of these as buses (Thanks Mrs. Groch). They carry electrons on them to the ETC. FADH2- They are a differ ...
Chapter 6, Section 3
... carbon atoms that are covalently bonded to other carbon atoms and other elements such as oxygen, hydrogen, and nitrogen. 1. Carbon forms bonds easily because it has 4 valence electrons. 2. Carbon atoms can bond to other carbon atoms, forming chains that are almost unlimited in length. 3. All living ...
... carbon atoms that are covalently bonded to other carbon atoms and other elements such as oxygen, hydrogen, and nitrogen. 1. Carbon forms bonds easily because it has 4 valence electrons. 2. Carbon atoms can bond to other carbon atoms, forming chains that are almost unlimited in length. 3. All living ...
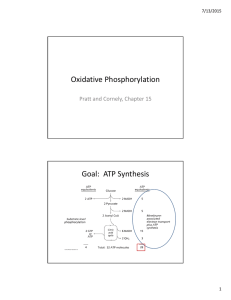
Oxidative Phosphorylation Goal: ATP Synthesis
... • In vivo, P/O ratio closer to 2.5 and 1.5 due to other proton “leaking” – i.e. importing phosphate ...
... • In vivo, P/O ratio closer to 2.5 and 1.5 due to other proton “leaking” – i.e. importing phosphate ...
signals - Biologie ENS
... Fuels: Molecules whose stored energy can be released for use. The most common fuel in organisms is glucose. Other molecules are first converted into glucose or other intermediate compounds. ...
... Fuels: Molecules whose stored energy can be released for use. The most common fuel in organisms is glucose. Other molecules are first converted into glucose or other intermediate compounds. ...
Cell Respiration powerpoint slides
... 1st part of reactions in cellular respiration - in the cytosol Breaking down glucose into two pyruvic acid molecules Released energy used to form ATP and NADH Consumes and releases ATP, needs the energy to start reaction ...
... 1st part of reactions in cellular respiration - in the cytosol Breaking down glucose into two pyruvic acid molecules Released energy used to form ATP and NADH Consumes and releases ATP, needs the energy to start reaction ...
Marine Mammal Dive Response
... A mitochondrion has two membranes. The inner membrane is highly folded, which greatly increase the membrane’s surface area. This improves the ability of the mitochondrion to do which of the following? A. move the cell through water B. digest metabolic wastes in the organelle C. convert solar energy ...
... A mitochondrion has two membranes. The inner membrane is highly folded, which greatly increase the membrane’s surface area. This improves the ability of the mitochondrion to do which of the following? A. move the cell through water B. digest metabolic wastes in the organelle C. convert solar energy ...
Cellular Respiration
... When a molecule of NAD+ (nicotinamide adenine dinucleotide) gains a hydrogen atom (not a hydrogen ion) the molecule becomes A) hydrogenated. B) oxidized. C) reduced. D) redoxed. E) a reducing agent. ...
... When a molecule of NAD+ (nicotinamide adenine dinucleotide) gains a hydrogen atom (not a hydrogen ion) the molecule becomes A) hydrogenated. B) oxidized. C) reduced. D) redoxed. E) a reducing agent. ...
Muscle Metabolism lecture teacher
... When meat is cooked, some of the proteins in it denature and become opaque, turning red meat pink. At 60 degrees C, the myoglobin itself denatures and becomes tan-coloured, giving well done meat a brownish-grey colour. Freezing for long periods of time can also denature the myoglobin. Finally, curin ...
... When meat is cooked, some of the proteins in it denature and become opaque, turning red meat pink. At 60 degrees C, the myoglobin itself denatures and becomes tan-coloured, giving well done meat a brownish-grey colour. Freezing for long periods of time can also denature the myoglobin. Finally, curin ...
Chapter 2 Notes ch._2_lecture_notes_2005
... Disaccharides are too large to pass through cell membranes and must be broken down to simple sugars through the process of hydrolysis. Polysaccharides: “Many Sugars” (polymers) Large insoluble molecules Ideal way to store potential chemical energy Starch: Energy storage polysaccharide formed by plan ...
... Disaccharides are too large to pass through cell membranes and must be broken down to simple sugars through the process of hydrolysis. Polysaccharides: “Many Sugars” (polymers) Large insoluble molecules Ideal way to store potential chemical energy Starch: Energy storage polysaccharide formed by plan ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑