Questions and answers from course Environmental microbiology on
... Oxidation: 2NH3 + 4H20 2 NO2- + 12 e- + 14 H+ Reduction: 3O2 + 12 e- + 12 H+ 6 H20 Overall reaction: 2NH3 + 3 O2 2 NO2- + 2 H20 + 2 H+ However hidden behind the equation is the important step of the ammonia monooxygenase to activate ammonia to hydroxylamine. This need of molecular oxygen is ...
... Oxidation: 2NH3 + 4H20 2 NO2- + 12 e- + 14 H+ Reduction: 3O2 + 12 e- + 12 H+ 6 H20 Overall reaction: 2NH3 + 3 O2 2 NO2- + 2 H20 + 2 H+ However hidden behind the equation is the important step of the ammonia monooxygenase to activate ammonia to hydroxylamine. This need of molecular oxygen is ...
Most common elements in living things are carbon, hydrogen
... oxygen (CHO). Proteins are made of carbon, hydrogen, oxygen, and nitrogen (CHON). Nucleic acids such as DNA and RNA contain carbon, hydrogen, oxygen, nitrogen, and phosphorus (CHON P). The four main classes of organic compounds (carbohydrates, lipids, proteins, and nucleic acids) that are essential ...
... oxygen (CHO). Proteins are made of carbon, hydrogen, oxygen, and nitrogen (CHON). Nucleic acids such as DNA and RNA contain carbon, hydrogen, oxygen, nitrogen, and phosphorus (CHON P). The four main classes of organic compounds (carbohydrates, lipids, proteins, and nucleic acids) that are essential ...
File
... • Essential nutrients cannot be synthesized in body – Minerals, most vitamins, eight amino acids, and one to three of the fatty acids must be consumed in diet ...
... • Essential nutrients cannot be synthesized in body – Minerals, most vitamins, eight amino acids, and one to three of the fatty acids must be consumed in diet ...
Biology 4974/5974, Evolution
... protocells, any major change would produce nonfunctional proteins. Thus, one code was favored and changes then were limited: frozen accident viewpoint. ...
... protocells, any major change would produce nonfunctional proteins. Thus, one code was favored and changes then were limited: frozen accident viewpoint. ...
Cell Respiration
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
chapter 9 cellular respiration: harvesting chemical energy
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
4.6 Fermentation
... chemical pathway that requires oxygen (why you breathe heavy after heavy excercise.) ...
... chemical pathway that requires oxygen (why you breathe heavy after heavy excercise.) ...
ATP ENERGY PRODUCTION - SHMD 339: Exercise Physiology 3
... cells of the body – muscles, liver When glycogen breaks down it releases pyruvic acid and energy. This energy is used to re-build ATP from ADP and P This system is anaerobic – no O2 Pyruvic acid is removed when O2 is available BUT: No O2 = Pyruvic acid is converted into lactic acid Muscles fail to c ...
... cells of the body – muscles, liver When glycogen breaks down it releases pyruvic acid and energy. This energy is used to re-build ATP from ADP and P This system is anaerobic – no O2 Pyruvic acid is removed when O2 is available BUT: No O2 = Pyruvic acid is converted into lactic acid Muscles fail to c ...
Introduction to Physiology: The Cell and General Physiology
... • ionized AA’s circulate in the plasma, ~ 35-65 mg/dl – control is not known, but even after a meal, plasma levels return to normal very rapidly – also, when plasma [AA] decreases, cell protein catabolism compensates ...
... • ionized AA’s circulate in the plasma, ~ 35-65 mg/dl – control is not known, but even after a meal, plasma levels return to normal very rapidly – also, when plasma [AA] decreases, cell protein catabolism compensates ...
Section 9–2 The Krebs Cycle and Electron Transport (pages 226–232)
... intermembrane space, making it positively charged. The other side of the membrane, from which those H+ ions have been taken, is now negatively charged. The charge differences that build up cause the ions to move. ...
... intermembrane space, making it positively charged. The other side of the membrane, from which those H+ ions have been taken, is now negatively charged. The charge differences that build up cause the ions to move. ...
( 2 points each).
... Multiple Choice. Choose the one alternative that best completes the statement or answers the question. ( 2 points each). ...
... Multiple Choice. Choose the one alternative that best completes the statement or answers the question. ( 2 points each). ...
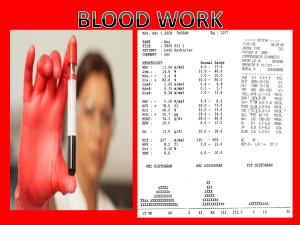
Blood Work - Mr. Lesiuk
... • Fasting blood sugar level tests are used to diagnose diabetes. • Blood work is collected first thing in the morning after 8 hours of fasting • Fasting, as the name suggests, means refraining from eating of drinking any liquids other than water for eight hours. It is used as a test for diabetes. I ...
... • Fasting blood sugar level tests are used to diagnose diabetes. • Blood work is collected first thing in the morning after 8 hours of fasting • Fasting, as the name suggests, means refraining from eating of drinking any liquids other than water for eight hours. It is used as a test for diabetes. I ...
File - King`s General Science
... and oxygen. Come from plants in the form of sugar (glucose) and starch (how plants store glucose). ...
... and oxygen. Come from plants in the form of sugar (glucose) and starch (how plants store glucose). ...
1 Chapter 8. Energy and energy transformations The chapter 8
... o Reaction 10: Enolphosphate has a high energy bond. It is hydrolyzed to form pyruvate with the synthesis of ATP. This irreversible reaction is catalyzed by the enzyme pyruvate kinase. - Fate of pyruvate depends on the availability of oxygen. o During the glycolysis, glucose is split into two 3 ...
... o Reaction 10: Enolphosphate has a high energy bond. It is hydrolyzed to form pyruvate with the synthesis of ATP. This irreversible reaction is catalyzed by the enzyme pyruvate kinase. - Fate of pyruvate depends on the availability of oxygen. o During the glycolysis, glucose is split into two 3 ...
Cellular respiration
... Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
... Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
printed handout sheets
... 9. Examples of key DNA binding proteins with pleiotropic effects. Tissue selective gene expression, and the selective responses to cytokines depend on specific DNA binding proteins which regulate gene expression. There may be literally thousands of these proteins, which are themselves expressed in a ...
... 9. Examples of key DNA binding proteins with pleiotropic effects. Tissue selective gene expression, and the selective responses to cytokines depend on specific DNA binding proteins which regulate gene expression. There may be literally thousands of these proteins, which are themselves expressed in a ...
20121016083538
... only used in cell that produces it only short term energy storage carbohydrates & fats are long term energy storage Whoa! Pass me the glucose & oxygen! ...
... only used in cell that produces it only short term energy storage carbohydrates & fats are long term energy storage Whoa! Pass me the glucose & oxygen! ...
Lecture 9: Biological Pathway Simulation
... We begin with a very simple imaginary metabolic network represented as a directed graph: ...
... We begin with a very simple imaginary metabolic network represented as a directed graph: ...
Cell Physiology
... – Contain oxidative enzymes – Enzymes remove H from various substrates and add it to O, producing hydrogen peroxide • catalase ...
... – Contain oxidative enzymes – Enzymes remove H from various substrates and add it to O, producing hydrogen peroxide • catalase ...
10/19
... NADH must be oxidized to NAD+ in order to oxidize glyceraldehyde-3-P In the absence of an electron transport chain pyruvate or a derivative serves as the electron acceptor for NADH Can lead to the production of some ATP ...
... NADH must be oxidized to NAD+ in order to oxidize glyceraldehyde-3-P In the absence of an electron transport chain pyruvate or a derivative serves as the electron acceptor for NADH Can lead to the production of some ATP ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑