Biochemistry http://www.brainpop.com/science/matterandchemistry
... Glycogen (in animals) – highly branched starch – glycogen. (In mammals, glycogen stored in liver and muscles provides a quick source of energy. – Excess glucose ? taken up from the blood - stored where ? ...
... Glycogen (in animals) – highly branched starch – glycogen. (In mammals, glycogen stored in liver and muscles provides a quick source of energy. – Excess glucose ? taken up from the blood - stored where ? ...
AP Bio Chapter 9: Cellular Respiration 1. What is the term for
... 2. The molecule that functions as the reducing agent (electron donor) in a redox or oxidation-reduction reaction a. gains electrons and gains energy. b. loses electrons and loses energy. c. gains electrons and loses energy. d. loses electrons and gains energy. e. neither gains nor loses electrons, b ...
... 2. The molecule that functions as the reducing agent (electron donor) in a redox or oxidation-reduction reaction a. gains electrons and gains energy. b. loses electrons and loses energy. c. gains electrons and loses energy. d. loses electrons and gains energy. e. neither gains nor loses electrons, b ...
Removal of materials from the blood
... • Liver suffers a disease which prevents its cells from absorbing bilirubin • Bile duct becomes blocked • Excessively high rate of red blood cells occurs ...
... • Liver suffers a disease which prevents its cells from absorbing bilirubin • Bile duct becomes blocked • Excessively high rate of red blood cells occurs ...
Homeostatic Control of Metabolism
... • Immediate use in energy production: nutrient pools • Synthesis into needed molecules (growth, maintenance) • Storage for later use • Fate depends on type of molecule: carbohydrate, protein, or fat ...
... • Immediate use in energy production: nutrient pools • Synthesis into needed molecules (growth, maintenance) • Storage for later use • Fate depends on type of molecule: carbohydrate, protein, or fat ...
Biochemical Pathways – Legends General Remarks for
... 32) The yeast system is shown here. The central SH group is written at the bottom, the marginal SH group at the top. In animals and in E. coli, the enzyme, E(SH)2, is replaced by acyl carrier protein, ACP. 33) Examples are GSH-homocystine transhydrogenase and protein disulfide reductase. 34) Reducti ...
... 32) The yeast system is shown here. The central SH group is written at the bottom, the marginal SH group at the top. In animals and in E. coli, the enzyme, E(SH)2, is replaced by acyl carrier protein, ACP. 33) Examples are GSH-homocystine transhydrogenase and protein disulfide reductase. 34) Reducti ...
U5Word
... 100GP-P/GPK-P = 5,000,000 activated GP/sec, rather than 1 Enzyme/1 effector. That's why it’s called the" amplification cascade". IV. Glucokinase (GK) 1. GK catalyzes G + ATP G6P + ADP, same as HXK. 2. GK is a liver enzyme; muscle doesn’t have it (has HXK). 3. The properties of GK are more suited t ...
... 100GP-P/GPK-P = 5,000,000 activated GP/sec, rather than 1 Enzyme/1 effector. That's why it’s called the" amplification cascade". IV. Glucokinase (GK) 1. GK catalyzes G + ATP G6P + ADP, same as HXK. 2. GK is a liver enzyme; muscle doesn’t have it (has HXK). 3. The properties of GK are more suited t ...
Cellular Respiration - Chandler Unified School District
... If the main purpose of cell respiration is to produce ATP, why do glycolysis & the Krebs cycle only make 4 molecules of ATP total by the time glucose has been converted to carbon dioxide? Although glycolysis & the Krebs cycle only produce 4 ATP molecules when glucose is converted to CO2 , these rea ...
... If the main purpose of cell respiration is to produce ATP, why do glycolysis & the Krebs cycle only make 4 molecules of ATP total by the time glucose has been converted to carbon dioxide? Although glycolysis & the Krebs cycle only produce 4 ATP molecules when glucose is converted to CO2 , these rea ...
I. Introduction to class
... cycle can start, pyruvic acid (3C) loses one carbon (as CO2) to become acetyl CoA (2C). Acetyl CoA (2C) joins oxaloacetic acid (4C) to form citric acid (6C). Cycle of 8 oxidation-reduction reactions that transfer energy to electron carrier molecules (coenzymes NAD+ and FAD). 2 molecules of car ...
... cycle can start, pyruvic acid (3C) loses one carbon (as CO2) to become acetyl CoA (2C). Acetyl CoA (2C) joins oxaloacetic acid (4C) to form citric acid (6C). Cycle of 8 oxidation-reduction reactions that transfer energy to electron carrier molecules (coenzymes NAD+ and FAD). 2 molecules of car ...
Bioloical Oxidation - Home
... between energy yielding processes or exergonic reactions •e.g. catabolism of glucose and fatty acids and energy requiring process or enderogenic reactions (e.g. anabolic pathways) A) catabolic reaction give energy , which can be stored in the form of ATP. B) Anabolic reactions which can utilize ener ...
... between energy yielding processes or exergonic reactions •e.g. catabolism of glucose and fatty acids and energy requiring process or enderogenic reactions (e.g. anabolic pathways) A) catabolic reaction give energy , which can be stored in the form of ATP. B) Anabolic reactions which can utilize ener ...
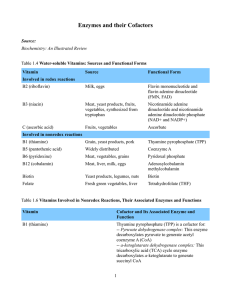
Enzymes and their Cofactors Source: Biochemistry: An Illustrated
... cofactors for: -- Methylmalonyl CoA mutase: This enzyme isomerizes methylmalonyl CoA into succinyl CoA in a reaction that is part of the pathway that degrades odd-numbered fatty acids -- Methionine synthase/homocysteine methyltransferase: This enzyme transfers a methyl group from 5-methyltetrahydrof ...
... cofactors for: -- Methylmalonyl CoA mutase: This enzyme isomerizes methylmalonyl CoA into succinyl CoA in a reaction that is part of the pathway that degrades odd-numbered fatty acids -- Methionine synthase/homocysteine methyltransferase: This enzyme transfers a methyl group from 5-methyltetrahydrof ...
Nutrients are chemical substances in food that provide energy, form
... reactions by increasing the frequency of collision, lowering activation energy, and properly orienting colliding molecules. A catalyst is a chemical substance that alters the rate of a chemical reaction without becoming part of the products of the reaction or being used up. Enzymes speed up chemical ...
... reactions by increasing the frequency of collision, lowering activation energy, and properly orienting colliding molecules. A catalyst is a chemical substance that alters the rate of a chemical reaction without becoming part of the products of the reaction or being used up. Enzymes speed up chemical ...
Quiz 6
... 1.Which of the following statements regarding ATP is/are correct? A) ATP serves as energy storage inside cells. B) ATP ultimately only does chemical work C) The regeneration of ATP from ADP and phosphate is a reaction that requires energy. D) A and B only 2. Which of the following is an example of p ...
... 1.Which of the following statements regarding ATP is/are correct? A) ATP serves as energy storage inside cells. B) ATP ultimately only does chemical work C) The regeneration of ATP from ADP and phosphate is a reaction that requires energy. D) A and B only 2. Which of the following is an example of p ...
FIGURE LEGENDS FIGURE 12.1 Glycolysis (Embden
... FIGURE LEGENDS FIGURE 12.1 Glycolysis (Embden-Meyerhof pathway). Glucose phosphorylation is regulated by hexokinase, an enzyme inhibited by glucose 6-phosphate. Glucose must be phosphorylated to glucose 6-phosphate to enter glycolysis or to be stored as glycogen. Two other important steps in the reg ...
... FIGURE LEGENDS FIGURE 12.1 Glycolysis (Embden-Meyerhof pathway). Glucose phosphorylation is regulated by hexokinase, an enzyme inhibited by glucose 6-phosphate. Glucose must be phosphorylated to glucose 6-phosphate to enter glycolysis or to be stored as glycogen. Two other important steps in the reg ...
b-Oxidation of fatty acids
... from anaerobic bacteria which were phagocytosed by eukaryote cells at the time oxygen appeared on earth, Similarities between mitochondria and bacteria include the presence of: • cardiolipin •transporters • ribosomes • circular RNA and DNA Therefore mitochondria protein synthesis should be inhibited ...
... from anaerobic bacteria which were phagocytosed by eukaryote cells at the time oxygen appeared on earth, Similarities between mitochondria and bacteria include the presence of: • cardiolipin •transporters • ribosomes • circular RNA and DNA Therefore mitochondria protein synthesis should be inhibited ...
Muscle Energy and Metabolism
... Muscle Metabolism (Cont) – Second metabolic pathway: aerobic respiration (Kreb’s Cycle / Citrus Acid Cycle) • Requires oxygen • produces much more ATP // glycolysis = 2 vs Kreb’s Cycle = 36 to 38 • less toxic end products CO2 // glycolysis produces lactic acid • Produces metabolic water • Reduces F ...
... Muscle Metabolism (Cont) – Second metabolic pathway: aerobic respiration (Kreb’s Cycle / Citrus Acid Cycle) • Requires oxygen • produces much more ATP // glycolysis = 2 vs Kreb’s Cycle = 36 to 38 • less toxic end products CO2 // glycolysis produces lactic acid • Produces metabolic water • Reduces F ...
ppt
... by dehydration synthesis reactions VARIABLE... 20 "letters" can make a very diverse "language" of words... ...
... by dehydration synthesis reactions VARIABLE... 20 "letters" can make a very diverse "language" of words... ...
Biochemistry Chapter 6
... Organic Chemistry = The element carbon is a component of almost all biological molecules. (Inorganic= no carbon) ...
... Organic Chemistry = The element carbon is a component of almost all biological molecules. (Inorganic= no carbon) ...
Energy Review True/False 1. A Cell that grow and reproduces
... 9. Light energy is trapped and converted into chemical energy during _____________________. 10. Photosynthesis occurs in the _________________. 11. __________ results from the removal of a phosphate group from ATP. 12. _______________is necessary for photosynthesis to occur and the most abundant of ...
... 9. Light energy is trapped and converted into chemical energy during _____________________. 10. Photosynthesis occurs in the _________________. 11. __________ results from the removal of a phosphate group from ATP. 12. _______________is necessary for photosynthesis to occur and the most abundant of ...
CHM 365 Name: Exam 3 Do all of the following 21 questions
... e) absolute requirement for ATP hydrolysis, no other energy source will work. ...
... e) absolute requirement for ATP hydrolysis, no other energy source will work. ...
PCGHS March Test ~ Year 2009 ~ Upper Six BIOLOGY Mark
... α-Ketoglutarate is converted to succinyl CoA (4C). ...
... α-Ketoglutarate is converted to succinyl CoA (4C). ...
4 Classes of Large Biological Molecules Carbohydrates Lipids
... Has two fatty acids attached to a glycerol molecule The 3rd –OH group is attached to a phosphate group (- charge) Show ambivalent properties toward water Steroids Have C skeletons consisting of 4 rings, only variation come in functional groups Cholesterol: precursor from which many other steroids ar ...
... Has two fatty acids attached to a glycerol molecule The 3rd –OH group is attached to a phosphate group (- charge) Show ambivalent properties toward water Steroids Have C skeletons consisting of 4 rings, only variation come in functional groups Cholesterol: precursor from which many other steroids ar ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑