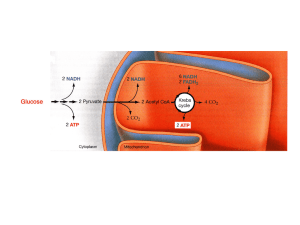
Chapter 9. Cellular Respiration STAGE 1: Glycolysis
... reactions. Glycolysis begins with the addition of energy. Two highenergy phosphates from two molecules of ATP are added to the six-carbon molecule glucose, producing a six-carbon molecule with two phosphates. ...
... reactions. Glycolysis begins with the addition of energy. Two highenergy phosphates from two molecules of ATP are added to the six-carbon molecule glucose, producing a six-carbon molecule with two phosphates. ...
146/18 = 8.1 ATP/carbon Atom. For Lauric acid
... from the porphyrin ring and saved in the iron-storage protein, ferritin, for later use. 28.42 Functional groups in biliverdin that are from oxidation: two carbon atoms at the top are oxidized from hydrocarbons to carbonyl groups. The carbon atom removed is oxidized to carbon monoxide. Functional gro ...
... from the porphyrin ring and saved in the iron-storage protein, ferritin, for later use. 28.42 Functional groups in biliverdin that are from oxidation: two carbon atoms at the top are oxidized from hydrocarbons to carbonyl groups. The carbon atom removed is oxidized to carbon monoxide. Functional gro ...
Dr. Walaa AL - Jedda – 2016 Metabolism of Glycogen Glycogen: is
... 2-Muscle glycogen on the other hand, is to act as readily available source of intermediates of glycolysis for provision of energy within the muscle itself. Muscle glycogen cannot directly contribute to blood glucose level. 3-Inherited deficiency of enzymes in the pathway of glycogen metabolism produ ...
... 2-Muscle glycogen on the other hand, is to act as readily available source of intermediates of glycolysis for provision of energy within the muscle itself. Muscle glycogen cannot directly contribute to blood glucose level. 3-Inherited deficiency of enzymes in the pathway of glycogen metabolism produ ...
Exam 3 - Chemistry Courses: About
... E. In addition to transketalases and transaldolases, the non-oxidative phase of the pentose phosphate pathway utilizes ___________________________ as enzymes. F. The pentose phosphate pathway is necessary to produce _____________ for fast-dividing cells and ____________________ for cells such as red ...
... E. In addition to transketalases and transaldolases, the non-oxidative phase of the pentose phosphate pathway utilizes ___________________________ as enzymes. F. The pentose phosphate pathway is necessary to produce _____________ for fast-dividing cells and ____________________ for cells such as red ...
Respiration Lab. eScience Lab 8. Experiments 1 and 2. Tips
... 6. Now compare your sucrose, starch, and milk (lactose) tubes to each control and describe the results for each below: Sucrose: The sucrose tube should also have a lot of carbon dioxide gas, because yeast has sucrase (the enzyme that metabolizes sucrose). There should be a similar amount of gas in ...
... 6. Now compare your sucrose, starch, and milk (lactose) tubes to each control and describe the results for each below: Sucrose: The sucrose tube should also have a lot of carbon dioxide gas, because yeast has sucrase (the enzyme that metabolizes sucrose). There should be a similar amount of gas in ...
Document
... is liberated, and H atoms removed are ultimately delivered to molecular oxygen, forming water. Some energy released is used to form ATP. Catabolic reactions Anabolic reactions © 2014 Pearson Education, Inc. ...
... is liberated, and H atoms removed are ultimately delivered to molecular oxygen, forming water. Some energy released is used to form ATP. Catabolic reactions Anabolic reactions © 2014 Pearson Education, Inc. ...
Unit Two “Energy Acquisition”
... 2. The “Phosphorylated” Glucose is split in two, forming 2 three-carbon sugar phosphates 3. In a series of reactions, both three-carbon sugars phosphate groups are converted to “Pyruvate” 4. The above Pyruvate forming process liberates Hydrogen so it can bond with NAD+ to form NADH, and 2 ATP’s are ...
... 2. The “Phosphorylated” Glucose is split in two, forming 2 three-carbon sugar phosphates 3. In a series of reactions, both three-carbon sugars phosphate groups are converted to “Pyruvate” 4. The above Pyruvate forming process liberates Hydrogen so it can bond with NAD+ to form NADH, and 2 ATP’s are ...
Macromolecule Expert Sheets
... 5. Name two common monosaccharides and give the molecular formula for each. Glucose and fructose both have the molecular formula C6H12O6. They are isomers. 6. What suffix is commonly found on the end of sugar names? -ose 7. How are monosaccharides used in a cell? They supply energy for cellular work ...
... 5. Name two common monosaccharides and give the molecular formula for each. Glucose and fructose both have the molecular formula C6H12O6. They are isomers. 6. What suffix is commonly found on the end of sugar names? -ose 7. How are monosaccharides used in a cell? They supply energy for cellular work ...
METABOLISM OF CARBOHYDRATES: GLYCOLYSIS
... enzyme lactase, which cleaves lactose into glucose and galactose. Microorganisms in the colon ferment undigested lactose to lactic acid generating methane (CH4) and hydrogen gas (H2). The gas produced creates the uncomfortable feeling of gut distention and the annoying problem of flatulence. The lac ...
... enzyme lactase, which cleaves lactose into glucose and galactose. Microorganisms in the colon ferment undigested lactose to lactic acid generating methane (CH4) and hydrogen gas (H2). The gas produced creates the uncomfortable feeling of gut distention and the annoying problem of flatulence. The lac ...
1. Why is the Krebs cycle so important in metabolism? The Krebs
... 1. Why is the Krebs cycle so important in metabolism? The Krebs cycle is the point of entry and exit of many critical molecules in respiration and metabolism. This includes all major calorie sources: carbohydrates, lipids, and proteins. 2. Why is oxygen necessary for the respiration of fat? Fatty ac ...
... 1. Why is the Krebs cycle so important in metabolism? The Krebs cycle is the point of entry and exit of many critical molecules in respiration and metabolism. This includes all major calorie sources: carbohydrates, lipids, and proteins. 2. Why is oxygen necessary for the respiration of fat? Fatty ac ...
05. Metabolism of carbohydrates 1
... enzyme lactase, which cleaves lactose into glucose and galactose. Microorganisms in the colon ferment undigested lactose to lactic acid generating methane (CH4) and hydrogen gas (H2). The gas produced creates the uncomfortable feeling of gut distention and the annoying problem of flatulence. The lac ...
... enzyme lactase, which cleaves lactose into glucose and galactose. Microorganisms in the colon ferment undigested lactose to lactic acid generating methane (CH4) and hydrogen gas (H2). The gas produced creates the uncomfortable feeling of gut distention and the annoying problem of flatulence. The lac ...
Pathways of Carbohydrate and Lipid Metabolism Glycolysis • Is the
... • The net result of the cycle is generation of 3 NADHs, 1 FADH2, and 1 GTP (GTP is sometimes interchangeably referred to as ATP because it is essentially converted to ATP very rapidly) ...
... • The net result of the cycle is generation of 3 NADHs, 1 FADH2, and 1 GTP (GTP is sometimes interchangeably referred to as ATP because it is essentially converted to ATP very rapidly) ...
Ch. 7 Cellular Respiration
... 2. The 6 carbon molecule that is formed is split into two three carbon molecules of PGAL. The 2 PGAL molecules are oxidized (each loses an electron) These electrons combine with NAD+ to form a new high energy compound called NADH (similar to NADP+) 3. The 4 phosphate groups that were added are now r ...
... 2. The 6 carbon molecule that is formed is split into two three carbon molecules of PGAL. The 2 PGAL molecules are oxidized (each loses an electron) These electrons combine with NAD+ to form a new high energy compound called NADH (similar to NADP+) 3. The 4 phosphate groups that were added are now r ...
Chapter 9
... •The ETC is a collection of molecules embedded in the cristae membrane of the mitochondrion. •There are thousands of copies of the ETC in every mitochondrion due to the extensive folding of cristae membrane. The ETC carries electrons delivered by NAD and FAD from glycolysis and the Krebs cycle to ox ...
... •The ETC is a collection of molecules embedded in the cristae membrane of the mitochondrion. •There are thousands of copies of the ETC in every mitochondrion due to the extensive folding of cristae membrane. The ETC carries electrons delivered by NAD and FAD from glycolysis and the Krebs cycle to ox ...
biology 110
... 4. What is phosporylation. What happens to the store of energy within a molecule when it phosphorylated? 5. What is an electron transport system? 6. Write out the formula for photosynthesis. Be sure to show how many molecules of each reactant and product are used or produced. 7. In question #6, whic ...
... 4. What is phosporylation. What happens to the store of energy within a molecule when it phosphorylated? 5. What is an electron transport system? 6. Write out the formula for photosynthesis. Be sure to show how many molecules of each reactant and product are used or produced. 7. In question #6, whic ...
aerobic respiration
... respiration all use glycolysis to oxidize glucose, but they differ in their final electron acceptor and whether an electron transport chain is used (respiration) or not (fermentation). ...
... respiration all use glycolysis to oxidize glucose, but they differ in their final electron acceptor and whether an electron transport chain is used (respiration) or not (fermentation). ...
Comments on metabolic needs for glucose and the role of
... stored in polymeric form (glycogen) is dictated by osmotic pressure considerations. That stored fat is about eight times more calorically dense than glycogen, when attendant water is factored in, accounts for the predominance of fat as a storage form of calories and, also, for the fact that ingested ...
... stored in polymeric form (glycogen) is dictated by osmotic pressure considerations. That stored fat is about eight times more calorically dense than glycogen, when attendant water is factored in, accounts for the predominance of fat as a storage form of calories and, also, for the fact that ingested ...
[j26]Chapter 5#
... ___ 13. Anaerobic respiration (or lactic acid fermentation) yields a net gain of two ATP molecules. ___ 14. Anaerobic respiration (or lactic acid fermentation) in the cell does not require the presence of oxygen in the conversion of one glucose molecule to two molecules of lactic acid. ___ 15. It is ...
... ___ 13. Anaerobic respiration (or lactic acid fermentation) yields a net gain of two ATP molecules. ___ 14. Anaerobic respiration (or lactic acid fermentation) in the cell does not require the presence of oxygen in the conversion of one glucose molecule to two molecules of lactic acid. ___ 15. It is ...
Topic Three Chemistry of Life - MrsGorukhomework
... * reduce – to gain an electron or hydrogen LEO says GER NH2 – Amino group COOH – carboxyl group – Main Organic Compounds 1. Carbohydrates – soluble in water, made up of C, H and O in a certain ratio. The monomer is called a saccharide. If only made up of one saccharide, called a monosaccharide. Many ...
... * reduce – to gain an electron or hydrogen LEO says GER NH2 – Amino group COOH – carboxyl group – Main Organic Compounds 1. Carbohydrates – soluble in water, made up of C, H and O in a certain ratio. The monomer is called a saccharide. If only made up of one saccharide, called a monosaccharide. Many ...
Anaerobic Respiration
... When the first step occurs and 2 acetaldehyde is formed, 2 CO₂ is released Then acetaldehyde accepts hydrogen and electrons from the 2 NADH formed through Glycolysis With the combining of e-, H+, and 2 acetaldehyde, 2 NAD+ is regenerated and 2ethanol is created ...
... When the first step occurs and 2 acetaldehyde is formed, 2 CO₂ is released Then acetaldehyde accepts hydrogen and electrons from the 2 NADH formed through Glycolysis With the combining of e-, H+, and 2 acetaldehyde, 2 NAD+ is regenerated and 2ethanol is created ...
Energy For Movement
... – This acidification discourages glycolysis – Decreases the muscle fibers’ calcium binding capacity and therefore impedes muscle contraction. ...
... – This acidification discourages glycolysis – Decreases the muscle fibers’ calcium binding capacity and therefore impedes muscle contraction. ...
Chapter 13 Carbohydrate Metabolism
... conditions, but the two ATPs produced from lactate fermentation are sufficient to sustain the life of anaerobic microorganisms. – In human metabolism, those two ATPs play a critical role by furnishing energy when cellular supplies of oxygen are insufficient for complete oxidation of pyruvate. – Duri ...
... conditions, but the two ATPs produced from lactate fermentation are sufficient to sustain the life of anaerobic microorganisms. – In human metabolism, those two ATPs play a critical role by furnishing energy when cellular supplies of oxygen are insufficient for complete oxidation of pyruvate. – Duri ...
some of Chapter 25
... recycling / breakdown cell growth / division store nutrients special jobs (secretion/contraction,…) ...
... recycling / breakdown cell growth / division store nutrients special jobs (secretion/contraction,…) ...
here - Sites@PSU
... Lactococcus sp. Lactobacillus sp. Leuconostoc sp. Pediococcus sp. Oenococcus sp. Streptococcus sp. Enterococcus sp. Sporolactobacillus sp. Carnobacterium sp. Aerococcus sp. Tetragenococcus sp. Vagococcus sp. Weisella sp. ...
... Lactococcus sp. Lactobacillus sp. Leuconostoc sp. Pediococcus sp. Oenococcus sp. Streptococcus sp. Enterococcus sp. Sporolactobacillus sp. Carnobacterium sp. Aerococcus sp. Tetragenococcus sp. Vagococcus sp. Weisella sp. ...
Glycogen Metabolism
... Phosphoglucomutase catalyzes the reversible reaction: glucose-1-phosphate glucose-6-phosphate ...
... Phosphoglucomutase catalyzes the reversible reaction: glucose-1-phosphate glucose-6-phosphate ...
Glucose

Glucose is a sugar with the molecular formula C6H12O6. The name ""glucose"" (/ˈɡluːkoʊs/) comes from the Greek word γλευκος, meaning ""sweet wine, must"". The suffix ""-ose"" is a chemical classifier, denoting a carbohydrate. It is also known as dextrose or grape sugar. With 6 carbon atoms, it is classed as a hexose, a sub-category of monosaccharides. α-D-glucose is one of the 16 aldose stereoisomers. The D-isomer (D-glucose) occurs widely in nature, but the L-isomer (L-glucose) does not. Glucose is made during photosynthesis from water and carbon dioxide, using energy from sunlight. The reverse of the photosynthesis reaction, which releases this energy, is a very important source of power for cellular respiration. Glucose is stored as a polymer, in plants as starch and in animals as glycogen.
















![[j26]Chapter 5#](http://s1.studyres.com/store/data/013325615_1-93c4a55e793afe312f3604738fdd639e-300x300.png)