Chapter 24 Fatty Acids as Energy Source Fatty Acids as Energy
... phytol in ruminant animals and thus appears in dairy products. ...
... phytol in ruminant animals and thus appears in dairy products. ...
Beta Oxidation of Fatty Acids
... the hydroxyl group at the beta position which forms a beta-ketoacyl-CoA derivative. This is the second oxidation step in this pathway and it is catalyzed by L-Hydroxyacyl-CoA Dehydrogenase. This enzyme needs to have NAD+ as a coenzyme and the NADH produced represents metabolic energy because for eve ...
... the hydroxyl group at the beta position which forms a beta-ketoacyl-CoA derivative. This is the second oxidation step in this pathway and it is catalyzed by L-Hydroxyacyl-CoA Dehydrogenase. This enzyme needs to have NAD+ as a coenzyme and the NADH produced represents metabolic energy because for eve ...
Review Problems #2 (Enzyme Review, Phosphatases
... We will definitely not get through all of these, but it is useful to have them in one place. 1) Outline the chemical intermediates in the degradation of the following amino acids: Asn, Asp. What cofactor(s) play a role in this process? What other end product may be formed from Asp. What cycle does t ...
... We will definitely not get through all of these, but it is useful to have them in one place. 1) Outline the chemical intermediates in the degradation of the following amino acids: Asn, Asp. What cofactor(s) play a role in this process? What other end product may be formed from Asp. What cycle does t ...
Health assessment of freshwater mussels using metabolomics
... Food limitation experiment Objective: Assess the metabolic changes in freshwater mussels brought into captivity and subjected to food limitation Hypothesis: Freshwater mussels held in captivity experience nutritional deficiency which will be exhibited by changes in metabolites associated with energ ...
... Food limitation experiment Objective: Assess the metabolic changes in freshwater mussels brought into captivity and subjected to food limitation Hypothesis: Freshwater mussels held in captivity experience nutritional deficiency which will be exhibited by changes in metabolites associated with energ ...
Incorporation of radioactive citrate into fatty acids
... Does the cytoplasmic cleavage of citrate represent an important extramitochondrial source of acetyl-CoA? The localization of 7 ° To of the cellular citrate in mitochondria is probably a reflection of the distribution of citrate-forming and citrateutilizing enzymesT; citrate-condensing activity is hi ...
... Does the cytoplasmic cleavage of citrate represent an important extramitochondrial source of acetyl-CoA? The localization of 7 ° To of the cellular citrate in mitochondria is probably a reflection of the distribution of citrate-forming and citrateutilizing enzymesT; citrate-condensing activity is hi ...
Chapter 18_CHEM 131
... • The lipids in membranes are phosphoglycerides, sphingomyelin, and cholesterol. • Lipids are organized in a lipid bilayer with hydrophobic (long carbon chain) portions inside and hydrophilic (polar groups) exposed to the water environment. • When a lipid bilayer is broken and the tails are exposed ...
... • The lipids in membranes are phosphoglycerides, sphingomyelin, and cholesterol. • Lipids are organized in a lipid bilayer with hydrophobic (long carbon chain) portions inside and hydrophilic (polar groups) exposed to the water environment. • When a lipid bilayer is broken and the tails are exposed ...
Biomolecule Notes
... Amino acids:joined by peptide bonds (dehydration synthesis again!!) Dipeptide (two amino acids) Polypeptide (many amino acids) ...
... Amino acids:joined by peptide bonds (dehydration synthesis again!!) Dipeptide (two amino acids) Polypeptide (many amino acids) ...
Cut and Paste Macromolecule Instructions
... 1. Describe the process of dehydration synthesis. 2. Give a biological function for each of the following molecules, with a named example of each: a. Carbohydrates b. Lipids 3. Where in a cell would long-chain carbohydrates be synthesized, and by what organelle? During what cellular process would th ...
... 1. Describe the process of dehydration synthesis. 2. Give a biological function for each of the following molecules, with a named example of each: a. Carbohydrates b. Lipids 3. Where in a cell would long-chain carbohydrates be synthesized, and by what organelle? During what cellular process would th ...
2.3 Carbon Compounds
... Carbon atoms can also bond to carbon atoms Carbon-carbon bonds can be single, double, or triple covalent bonds Chains of carbon atoms can also form rings. ...
... Carbon atoms can also bond to carbon atoms Carbon-carbon bonds can be single, double, or triple covalent bonds Chains of carbon atoms can also form rings. ...
2.3_Carbon_Compounds
... Carbon atoms can also bond to carbon atoms Carbon-carbon bonds can be single, double, or triple covalent bonds Chains of carbon atoms can also form rings. ...
... Carbon atoms can also bond to carbon atoms Carbon-carbon bonds can be single, double, or triple covalent bonds Chains of carbon atoms can also form rings. ...
Biomolecules
... • Phosphate group (head) is polar and water soluble (hydrophilic) • Two fatty acid tails are hydrophobic •This allows the phospholipids to form bilayers and membranes ...
... • Phosphate group (head) is polar and water soluble (hydrophilic) • Two fatty acid tails are hydrophobic •This allows the phospholipids to form bilayers and membranes ...
Biochemistry PP
... form polymers is called Dehydration synthesis (removing water, putting together) – For each bond, a water molecule needs to be pulled out to join the 2 monomers together. – It is a building up process, going from simple to more ...
... form polymers is called Dehydration synthesis (removing water, putting together) – For each bond, a water molecule needs to be pulled out to join the 2 monomers together. – It is a building up process, going from simple to more ...
1. Identify the structural formula. Use these choices - burgess
... For each statement, write the letter of one of the structural formulas in number 1 above. A letter can be used more than once. _B_ 2. When many are bonded together, a protein is formed. _C_ 3. It is a disaccharide with the formula C12H22O11 _A_ 4. It is an isomer of fructose and galactose [each have ...
... For each statement, write the letter of one of the structural formulas in number 1 above. A letter can be used more than once. _B_ 2. When many are bonded together, a protein is formed. _C_ 3. It is a disaccharide with the formula C12H22O11 _A_ 4. It is an isomer of fructose and galactose [each have ...
Lipids lecture(5) by Prof.Dr.Moaed Al
... As the degree of unsaturation increases, fatty acids become more fluid--> melting point decreases ( kinks in tails decrease number of van der Waals interactions). Fatty acids are also an important sources of energy. 9 kcal/g vs. 4 kcal/g for carbohydrates and proteins. ...
... As the degree of unsaturation increases, fatty acids become more fluid--> melting point decreases ( kinks in tails decrease number of van der Waals interactions). Fatty acids are also an important sources of energy. 9 kcal/g vs. 4 kcal/g for carbohydrates and proteins. ...
Introduction Fatty acid biosynthesis is one of the most
... Subsystem: Fatty Acid Biosynthesis FASII Andrei Osterman1,2 1The Burnham Institute, 2FIG ...
... Subsystem: Fatty Acid Biosynthesis FASII Andrei Osterman1,2 1The Burnham Institute, 2FIG ...
Lactic Acid and Energy from Fats and Proteins
... Fatty acids = types of fat found in muscle cells and adipose tissue that are used for energy Fatty acids are stored in the body as triglycerides Lipolysis = process where triglycerides are broken down and the resulting fatty acids become available to be used as an energy ...
... Fatty acids = types of fat found in muscle cells and adipose tissue that are used for energy Fatty acids are stored in the body as triglycerides Lipolysis = process where triglycerides are broken down and the resulting fatty acids become available to be used as an energy ...
NUTRITION - Purdue University
... 3 carbon atoms = propionic acid CH3CH2COOH 4 carbon atoms = butyric acid CH3CH2CH2COOH ...
... 3 carbon atoms = propionic acid CH3CH2COOH 4 carbon atoms = butyric acid CH3CH2CH2COOH ...
Inorganic Chemistry PP
... • Bonds that form between amino acids • Dipeptide bond: formed between 2 amino acids • Polypeptide bond: formed between more than 2 amino acids ...
... • Bonds that form between amino acids • Dipeptide bond: formed between 2 amino acids • Polypeptide bond: formed between more than 2 amino acids ...
Lecture 35 - Lipid Metabolism 1
... -oxidation is a chemical source of water for desert animals Besides the payout of ATP that comes from fatty acid oxidation, another benefit is the generation of H2O that occurs when O2 is reduced by the final reaction in the electron transport system, as well as, the formation of H2O in oxidative ...
... -oxidation is a chemical source of water for desert animals Besides the payout of ATP that comes from fatty acid oxidation, another benefit is the generation of H2O that occurs when O2 is reduced by the final reaction in the electron transport system, as well as, the formation of H2O in oxidative ...
Biochemistry File - Northwest ISD Moodle
... 4. Proteins – polymers of amino acids joined by peptide bonds Used to build cells, transport molecules, and control the rate of reactions Made of “C”, “H”, “O”, and “N” 20 different amino acids ...
... 4. Proteins – polymers of amino acids joined by peptide bonds Used to build cells, transport molecules, and control the rate of reactions Made of “C”, “H”, “O”, and “N” 20 different amino acids ...
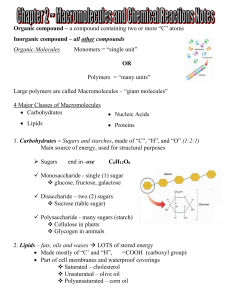
Organic Molecules: The Molecules of Life
... Fats- found in animals We can think of body fat and the derived products of lard and butter Oils are derived from plants. Ie) olive oil, canola oil…. Made of a glycerol backbone that has 3 fatty acid chains attached to it. Think of a big letter E. Fatty acids are chains of Carbon (16-18 or so) have ...
... Fats- found in animals We can think of body fat and the derived products of lard and butter Oils are derived from plants. Ie) olive oil, canola oil…. Made of a glycerol backbone that has 3 fatty acid chains attached to it. Think of a big letter E. Fatty acids are chains of Carbon (16-18 or so) have ...
CHAPTER-IV LIPID METABOLISM BETA
... point off regulation in saturated straight-cchain fatty acid syntheesis, and is subject to both phosphorrylation and d allosteric regulation. Regulation by phosphhorylation occurs o mostly in mammals, while allosteric reguulation occurrs in most organisms. Allosteric control c occuurs as feedbackk i ...
... point off regulation in saturated straight-cchain fatty acid syntheesis, and is subject to both phosphorrylation and d allosteric regulation. Regulation by phosphhorylation occurs o mostly in mammals, while allosteric reguulation occurrs in most organisms. Allosteric control c occuurs as feedbackk i ...
Chapter 5 - SchoolRack
... Made of glycerol and 3 fatty acids Fatty acid has a long carbon skeleton and a carboxyl group C-H bonds responsible for hydrophobia of fats ...
... Made of glycerol and 3 fatty acids Fatty acid has a long carbon skeleton and a carboxyl group C-H bonds responsible for hydrophobia of fats ...
Fatty acid synthesis

Fatty acid synthesis is the creation of fatty acids from acetyl-CoA and malonyl-CoA precursors through action of enzymes called fatty acid synthases. It is an important part of the lipogenesis process, which – together with glycolysis – functions to create fats from blood sugar in living organisms.