Improved Synthesis, Separation, Transition Metal Coordination and
... For higher temperatures the NMR tube was tube pressurized to 90 psi with O2 ............................................................................... 59 H spectra of meso-Ni2Cl4(et,ph-P4) with 1-hexene in acetone-d6/D2O solution recorded at 100°C, tube pressurized 90 psi of O2 .............. 5 ...
... For higher temperatures the NMR tube was tube pressurized to 90 psi with O2 ............................................................................... 59 H spectra of meso-Ni2Cl4(et,ph-P4) with 1-hexene in acetone-d6/D2O solution recorded at 100°C, tube pressurized 90 psi of O2 .............. 5 ...
Recent advances in homogeneous nickel catalysis
... of hydrolysis. However, their reactivity towards oxidative addition by metals is also considerably reduced, often leading to the need for harsher reaction conditions. For these reasons, catalyst systems capable of activating the C–O bonds of functional groups other than triflates—such as ethers, est ...
... of hydrolysis. However, their reactivity towards oxidative addition by metals is also considerably reduced, often leading to the need for harsher reaction conditions. For these reasons, catalyst systems capable of activating the C–O bonds of functional groups other than triflates—such as ethers, est ...
PHENOL - Gneet's
... The above reaction is laboratory method for preparation of phenol 2. Hydrolysis of diazonium salt ...
... The above reaction is laboratory method for preparation of phenol 2. Hydrolysis of diazonium salt ...
DISTINGUISH TESTS
... In a molecule if Carbon atom is surrounded by four different groups called asymmetric carbon or chiral carbon which is must for optical active compound. 1:1 ratio of dextro and leavo mixture is known as racemic mixture. The process of conversion of enantiomer into a racemic mixture is known as ...
... In a molecule if Carbon atom is surrounded by four different groups called asymmetric carbon or chiral carbon which is must for optical active compound. 1:1 ratio of dextro and leavo mixture is known as racemic mixture. The process of conversion of enantiomer into a racemic mixture is known as ...
The bite angle makes the catalyst
... Rhodium catalysed hydroformylation of alkenes is a mild and clean method for the functionalization of hydrocarbons. The atom economy of the reaction is 100% and the selectivity for the desired aldehyde can be very high. Hydroformylation of alkenes is one of the most important homogeneously catalysed ...
... Rhodium catalysed hydroformylation of alkenes is a mild and clean method for the functionalization of hydrocarbons. The atom economy of the reaction is 100% and the selectivity for the desired aldehyde can be very high. Hydroformylation of alkenes is one of the most important homogeneously catalysed ...
74 CHAPTER-IV "LEAD (IV) ACETATE OXIDATIONS"
... Industriesweige,' vol14, p 372; Springer, Berlin, 1921-1925; K. Kindler, and W. Psechke, Arch. Pharm., 1932, 270, 340, 347.; 1934, 272, 236; K. Kindler, and T. Li, Ber. Deut. chem. Ges., 1941, 74, 321. 45. A. Me. Killop, B. P. Swann, E. C. Taylor, J. Am. Chem. Soc., 1971, 93, ...
... Industriesweige,' vol14, p 372; Springer, Berlin, 1921-1925; K. Kindler, and W. Psechke, Arch. Pharm., 1932, 270, 340, 347.; 1934, 272, 236; K. Kindler, and T. Li, Ber. Deut. chem. Ges., 1941, 74, 321. 45. A. Me. Killop, B. P. Swann, E. C. Taylor, J. Am. Chem. Soc., 1971, 93, ...
unit 12 aldehydes, ketones and carboxylic acids
... Q.24 Why the oxidation of toluene to benzaldehyde with CrO3 is carried out in the presence of acetic anhydride. Ans If acetic anhydride is not used we will get benzoic acid.Acetic anhydride used to prevent oxidation of benzaldehyde to benzoic acid. Q.25 Melting point of an acid with even no. of carb ...
... Q.24 Why the oxidation of toluene to benzaldehyde with CrO3 is carried out in the presence of acetic anhydride. Ans If acetic anhydride is not used we will get benzoic acid.Acetic anhydride used to prevent oxidation of benzaldehyde to benzoic acid. Q.25 Melting point of an acid with even no. of carb ...
The catalytic function of a silica gel-immobilized Mn(II)
... range of alkenes [6]. Manganese porphyrin complexes have been extensively investigated as models for enzymes and used in catalysis with various single oxygen atom donors including H2 O2 [7]. Alkene epoxidation reactions catalyzed by manganese complexes in homogeneous and heterogeneous media and the ...
... range of alkenes [6]. Manganese porphyrin complexes have been extensively investigated as models for enzymes and used in catalysis with various single oxygen atom donors including H2 O2 [7]. Alkene epoxidation reactions catalyzed by manganese complexes in homogeneous and heterogeneous media and the ...
ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO …
... As we have already seen, substituted amines can react with aldehydes and ketones to form a variety of products. ...
... As we have already seen, substituted amines can react with aldehydes and ketones to form a variety of products. ...
Kinetic modelling of the Maillard reaction between proteins and sugars
... 1.3 Sugar degradation ...
... 1.3 Sugar degradation ...
phenols - Gneet`s
... Sodium salt of aryl sulphonic acids on fusion with sodium hydroxide at 300-350oC yield phenol ...
... Sodium salt of aryl sulphonic acids on fusion with sodium hydroxide at 300-350oC yield phenol ...
Amidations of Rosin with Isocyanates
... Based on the comparison of the data in Table 3 with those in Tables 1 and 2, it is found that the amidation of rosin with phenyl isocyanate could also proceeded well, but a little slower rate than that of abietic acid with the isocyanate. For example, rosin and abietic acid reacted with phenyl isocy ...
... Based on the comparison of the data in Table 3 with those in Tables 1 and 2, it is found that the amidation of rosin with phenyl isocyanate could also proceeded well, but a little slower rate than that of abietic acid with the isocyanate. For example, rosin and abietic acid reacted with phenyl isocy ...
amine cured-epoxy matrices
... utilized as curing agents in epoxy matrices for high performance composites. This produces a heteropolymer consisting of epoxy molecules linked together through the reactive sites of the curing agent. Usually when it is stated that an epoxy matrix is amine cured, it is meant that the curing agent(s) ...
... utilized as curing agents in epoxy matrices for high performance composites. This produces a heteropolymer consisting of epoxy molecules linked together through the reactive sites of the curing agent. Usually when it is stated that an epoxy matrix is amine cured, it is meant that the curing agent(s) ...
Isoindolone Formation via Intramolecular Diels
... with processing which appeared suitable for long-term commercial manufacture, as shown in Scheme 2. The reaction was performed successfully at 100 L scale to produce approximately 14 kg of amidoxime 5 in total. To utilise this amidoxime fragment, it was important then to find a suitable commercial sy ...
... with processing which appeared suitable for long-term commercial manufacture, as shown in Scheme 2. The reaction was performed successfully at 100 L scale to produce approximately 14 kg of amidoxime 5 in total. To utilise this amidoxime fragment, it was important then to find a suitable commercial sy ...
Organic Synthesis II
... Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ...
... Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ...
9: Formation of Alkenes and Alkynes. Elimination Reactions
... The C-A and C-B bonds break in the elimination reaction, and a second bond forms between the two C's to form a C=C bond. "A-B" in Figure 9.01 may not be an actual reaction product, but ...
... The C-A and C-B bonds break in the elimination reaction, and a second bond forms between the two C's to form a C=C bond. "A-B" in Figure 9.01 may not be an actual reaction product, but ...
Organic Chemistry - UCR Chemistry
... and compounds related to them. These are ionic reactions in which one group on the molecule (a leaving group) is replaced by another group (a nucleophile). The transformation of haloalkanes (R-X) into alcohols (R-OH) where an OH group replaces the halogen (X) is an example of nucleophilic substituti ...
... and compounds related to them. These are ionic reactions in which one group on the molecule (a leaving group) is replaced by another group (a nucleophile). The transformation of haloalkanes (R-X) into alcohols (R-OH) where an OH group replaces the halogen (X) is an example of nucleophilic substituti ...
Chapter 19
... Amine salts are also used to catalyze a variety of organic reactions that feature two components that are soluble in different liquid phases (e.g. organic and aqueous) ...
... Amine salts are also used to catalyze a variety of organic reactions that feature two components that are soluble in different liquid phases (e.g. organic and aqueous) ...
Handout VI
... reaction and the equilibrium can be shifted to the right by using an acid or base as catalyst. In this case the rate limiting step is the attack of the nucleophile. This reaction proceeds quantitatively for aldehydes and simple ketones but not so for aryl alkyl ketones and diaryl ketones (Scheme 11) ...
... reaction and the equilibrium can be shifted to the right by using an acid or base as catalyst. In this case the rate limiting step is the attack of the nucleophile. This reaction proceeds quantitatively for aldehydes and simple ketones but not so for aryl alkyl ketones and diaryl ketones (Scheme 11) ...
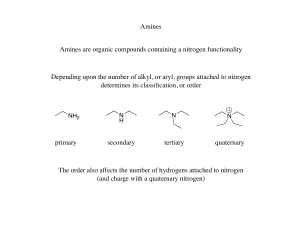
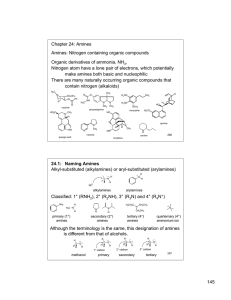
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Table 24.1 (p. 899): pKa values of ammonium ions Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.25) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably less basic than alkyl amines (pKa ~ 5 or less). The nitr ...
... Table 24.1 (p. 899): pKa values of ammonium ions Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.25) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably less basic than alkyl amines (pKa ~ 5 or less). The nitr ...
Organic synthesis and methodology related to the malaria drug artemisinin
... discussed fully. Key features of the syntheses will include alkylation of menthone derivatives using Noyori’s zincate enolate method and nucleophilic addition to a hindered ketone using either organocerium or acetylide nucleophiles. In addition, two alternative olefin metathesis approaches are descr ...
... discussed fully. Key features of the syntheses will include alkylation of menthone derivatives using Noyori’s zincate enolate method and nucleophilic addition to a hindered ketone using either organocerium or acetylide nucleophiles. In addition, two alternative olefin metathesis approaches are descr ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.