* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download review-rough
Asymmetric induction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Homoaromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
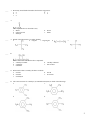
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. ____ 2. a. ____ What is the monomer for the polymer Kel-F shown below? b. c. d. 3. Which one of the following contains compounds from which a condensation polymer can be produced? a. and b. and c. and d. and ____ ____ 4. An amine is characterized by what functional group? a. -CO2CH3 d. b. -NH2 e. c. -CO2H 5. Which of the following is an alcohol? a. NaOH b. -CHO -OH d. e. c. ____ 6. When two alcohols undergo a self condensation, what is formed? a. liquid alcohol d. an aldehyde b. a ketone e. an ether c. an ester 1 ____ 7. How many actual double bonds does the benzene ring possess? a. 4 d. 1 b. 3 e. 0 c. 2 ____ 8. The compound above is classified as a(n) a. alkane b. carboxylic acid c. aldehyde ____ ____ d. e. 9. Which of the following is a secondary alcohol? a. b. CH3OH c. CH3CH2OH ____ d. e. 10. What is the correct name for the above compound? a. 2-methyl-3-butanol d. b. 2-pentanol e. c. isobutanol ____ ketone alkene 11. What results when a secondary alcohol is oxidized? a. a ketone d. b. an amine e. c. an aldehyde 3-methyl-2-butanol none of these an acid no reaction 12. The correct structure for 1-methyl-2,4,6-trinitrobenzene(TNT) is which of the following? a. c. b. d. 2 ____ 13. The formula for methyl ethanoate is which of the following? a. c. b. ____ d. 14. The correct IUPAC name for the compound above is which of the following? a. 5-ol-2-ethylpentane ether c. 3-propoxypentanal b. 2-ethoxy-5-pentanone d. 4-ethoxypentanal ____ ____ 15. Which type of reaction will an alkene not undergo? a. addition d. b. polymerization e. c. oxidation dehydration hydration 16. Which group of these compounds tends to be the most reactive? a. alkanes c. alkynes b. alkenes d. aromatics Completion Complete each sentence or statement. 17. Kevlar, a strong polymer used in bullet proof vests, is made by the following condensation of monomers: and The structure of the polymer Kevlar is ___________________________________. 18. The monomer that must be used to produce the polymer given below is ____________________. Matching Match each of the following terms with its appropriate description. a. aspartame e. triglyceride b. termination f. polyethene c. hydrolysis g. hydroxyl d. nylon 6,6 3 ____ 19. A polymer made of many ethene molecules linked together ____ 20. The last stage of the addition polymerization reaction when two unpaired electron ends combine is called: ____ 21. A substitute for silk, this substance is formed from the reaction of adipic acid with 1,6 diaminohexane. ____ 22. This artificial sweetener is thought to be dangerous because it releases methanol as one of its breakdown products. Experts feel it is not in high enough concentration to be dangerous. ____ 23. The type of reaction whereby lactose is broken down into galactose and glucose ____ 24. This specific group links the phosphate on one monomer and the ribose of another monomer by condensation reactions. ____ 25. The immiscibility of fats and oils in water is due to the non-polar nature of this type of molecule. Short Answer 26. The structure given below is a polymer classified as a polyamide. This polymer is stable in temperatures above 350°C, and can be used as a protective coating on hot surfaces. What monomers were used to make this polyamide? 27. In the design of a new baby diaper, the manufacturer uses two polymers. The structure of these molecules is given below. Which polymer is best suited to the outside of the diaper and which to the inside? , (I) (II) 4 orgo Answer Section MULTIPLE CHOICE 1. ANS: B 2. ANS: C 3. ANS: D 4. ANS: B 5. ANS: D 6. ANS: E 7. ANS: E 8. ANS: C 9. ANS: C 10. ANS: D 11. ANS: A 12. ANS: A 13. ANS: D 14. ANS: D 15. ANS: D 16. ANS: C COMPLETION 17. ANS: 18. ANS: MATCHING 19. ANS: F 20. ANS: B 21. ANS: D 22. ANS: A 23. ANS: C 5 24. ANS: G 25. ANS: E SHORT ANSWER 26. ANS: - is a polyamide polymer - monomers are: , 27. ANS: - (II) is hydrophobic (non-polar), so it would prevent leakage of water to the outside - (I) will allow water to mix with the lining to absorb water - (I) should be on the inside - (II) should be on the outside 6