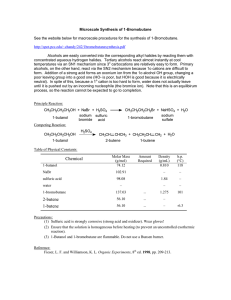
Alcohols from Alkenes: Oxymercuration–Demercuration
... form of the starting material reacts in such a way that it gives a specific stereoisomeric form of the product. ...
... form of the starting material reacts in such a way that it gives a specific stereoisomeric form of the product. ...
Carbene Singlets, Triplets, and the Physics that
... Unoccupied Molecular Orbital (abbreviated HOMO and LUMO respectively). The energy difference between these orbitals, often referred to as the HOMO-LUMO gap, is what determines bonding in most circumstances where orbital mixing is a valid approximation in general. The HOMO-LUMO gap determines the var ...
... Unoccupied Molecular Orbital (abbreviated HOMO and LUMO respectively). The energy difference between these orbitals, often referred to as the HOMO-LUMO gap, is what determines bonding in most circumstances where orbital mixing is a valid approximation in general. The HOMO-LUMO gap determines the var ...
Modules 261 12th edition
... The (E) – (Z) System for Designating Alkene Diastereomers How to Use the (E) –(Z) System Relative Stabilities of Alkenes - heat of reaction - overall relative stabilities of alkenes Cycloalkenes Synthesis of Alkenes: Elimination Reactions Dehydrohalogenation of Alkyl Halides - Bases Used in Dehydroh ...
... The (E) – (Z) System for Designating Alkene Diastereomers How to Use the (E) –(Z) System Relative Stabilities of Alkenes - heat of reaction - overall relative stabilities of alkenes Cycloalkenes Synthesis of Alkenes: Elimination Reactions Dehydrohalogenation of Alkyl Halides - Bases Used in Dehydroh ...
Reaction of Alkenes
... Reaction of Alkenes: HX Addition Markovnikov’s Rule: In electrophilic addition reactions of M k ik ’ R l I l t hili dditi ti f alkenes, the electrophile adds to the least substituted carbon giving rise to the more stable intermediate. Example: Addition of HX Markovnikov Addition (only product) ...
... Reaction of Alkenes: HX Addition Markovnikov’s Rule: In electrophilic addition reactions of M k ik ’ R l I l t hili dditi ti f alkenes, the electrophile adds to the least substituted carbon giving rise to the more stable intermediate. Example: Addition of HX Markovnikov Addition (only product) ...
Document
... • There are seven f orbitals per n level. – The f orbitals have complicated names. – They have an = 3 – m = -3,-2,-1,0,+1,+2, +3 7 values of m – The f orbitals have important effects in the lanthanide and actinide ...
... • There are seven f orbitals per n level. – The f orbitals have complicated names. – They have an = 3 – m = -3,-2,-1,0,+1,+2, +3 7 values of m – The f orbitals have important effects in the lanthanide and actinide ...
Molecular Orbitals - Calderglen High School
... A substitution reaction is the reaction in which an atom or group of atoms in a molecule is replaced by another atom or group of atoms. The initiation step in the reaction between methane and chlorine is slow and is used to determine the rate of the reaction. Similar reactions occur with other halog ...
... A substitution reaction is the reaction in which an atom or group of atoms in a molecule is replaced by another atom or group of atoms. The initiation step in the reaction between methane and chlorine is slow and is used to determine the rate of the reaction. Similar reactions occur with other halog ...
CH8
... 3. An electron dot structure can be used to show the shared pair of electrons of the covalent bond. 4. Using page 218 use electron dots to combine two Fluorine atoms then show the electron configuration for each atom. 5. Structural Formula – represents the covalent bonds by using dashes, each dash r ...
... 3. An electron dot structure can be used to show the shared pair of electrons of the covalent bond. 4. Using page 218 use electron dots to combine two Fluorine atoms then show the electron configuration for each atom. 5. Structural Formula – represents the covalent bonds by using dashes, each dash r ...
Chemistry 201 C Alkenes
... 2 sp2 orbitals, one from each carbon, overlap to form a σ-bond. 2 p orbitals, one from each carbon, overlap to form a π (pi) bond. ...
... 2 sp2 orbitals, one from each carbon, overlap to form a σ-bond. 2 p orbitals, one from each carbon, overlap to form a π (pi) bond. ...
Synthesis of 1
... min, decant the dried liquid into the dry 5-mL round-bottomed flask. This flask is conveniently dried by rinsing it with a milliliter of ethanol followed by a milliliter of acetone and then drawing air through it using the vacuum. It is very important when drying an apparatus of this type to remove ...
... min, decant the dried liquid into the dry 5-mL round-bottomed flask. This flask is conveniently dried by rinsing it with a milliliter of ethanol followed by a milliliter of acetone and then drawing air through it using the vacuum. It is very important when drying an apparatus of this type to remove ...
General Chemistry Unit 11
... 1) Determine the mass of the empty crucible and record in table 1. 2) Obtain a 6 in strip of Mg ribbon. Cut it into about 12 pieces and put the pieces into the crucible and determine the mass. Record in table 1. 3) Place the crucible on the clay triangle and slowly heat it with a “soft” blue flame f ...
... 1) Determine the mass of the empty crucible and record in table 1. 2) Obtain a 6 in strip of Mg ribbon. Cut it into about 12 pieces and put the pieces into the crucible and determine the mass. Record in table 1. 3) Place the crucible on the clay triangle and slowly heat it with a “soft” blue flame f ...
Relative Reactivity of Aldehydes and Ketones: Generally
... Acidic conditions use neutral water, H2O, as the nucleophile… Neutral carbonyl and neutral water aren’t so very attracted to each other… The oxygen atom in water is electronegative and less willing to share its electron density to form a bond to the carbonyl. We must tweak the system to make them mo ...
... Acidic conditions use neutral water, H2O, as the nucleophile… Neutral carbonyl and neutral water aren’t so very attracted to each other… The oxygen atom in water is electronegative and less willing to share its electron density to form a bond to the carbonyl. We must tweak the system to make them mo ...
Partial Periodic Table - Organic Chemistry at CU Boulder
... c) Circle the more stable conformation in part 3b. If the two conformations have the same energy, label then “same.” ...
... c) Circle the more stable conformation in part 3b. If the two conformations have the same energy, label then “same.” ...
Dissertation:
... activity in PLLA and L-LA alcoholysis reactions and allow to obtain alkyl lactates under mild conditions, in a shorter period of time and with higher yield. Studies of alcoholysis reaction in which ethyl lactate (hydroxyester) was used both as a solvent and reactant were also carried out and allowed ...
... activity in PLLA and L-LA alcoholysis reactions and allow to obtain alkyl lactates under mild conditions, in a shorter period of time and with higher yield. Studies of alcoholysis reaction in which ethyl lactate (hydroxyester) was used both as a solvent and reactant were also carried out and allowed ...
CH 12-3 Power Point
... •Important multi-step synthesis for making new C-C bonds, and 1o, 2o, and 3o alcohols. ...
... •Important multi-step synthesis for making new C-C bonds, and 1o, 2o, and 3o alcohols. ...
chapter 4 review_package
... When 51.0 grams of NH3 is burned in an excess of oxygen, 52.65 g of water are produced. i. Calculate the theoretical yield of H2O. ...
... When 51.0 grams of NH3 is burned in an excess of oxygen, 52.65 g of water are produced. i. Calculate the theoretical yield of H2O. ...
lecture slides of chap19_FU
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.