Organometallic Organometallic Chemistry
... Valence shells of a MT can accommodate 18 electrons: 2 in each of the five d orbitals (10 in total); 2 in each of the three p orbitals (6 in total); and 2 in the s orbital. Combination of these atomic orbitals with ligand orbitals: 9 MOs which are either metal-ligand bonding or non-bonding. (There a ...
... Valence shells of a MT can accommodate 18 electrons: 2 in each of the five d orbitals (10 in total); 2 in each of the three p orbitals (6 in total); and 2 in the s orbital. Combination of these atomic orbitals with ligand orbitals: 9 MOs which are either metal-ligand bonding or non-bonding. (There a ...
Chapter 11: Reactions of Alkyl Halides There are two basic types of
... A Walden cycle is considered to be any series of reactions that converts one enantiomer into its mirror image. A series of studies took off in the 1920s and 1930s, with one of the first being the conversion of (+)-1-phenyl-2-propanol to (-)-1-phenyl-2propanol. Originally it was thought that the same ...
... A Walden cycle is considered to be any series of reactions that converts one enantiomer into its mirror image. A series of studies took off in the 1920s and 1930s, with one of the first being the conversion of (+)-1-phenyl-2-propanol to (-)-1-phenyl-2propanol. Originally it was thought that the same ...
Bent`s Rule
... hydrogen substituents. This simple system demonstrates that hybridised atomic orbitals with higher p character will have a smaller angle between them. ...
... hydrogen substituents. This simple system demonstrates that hybridised atomic orbitals with higher p character will have a smaller angle between them. ...
T. V. RajanBabu Chemistry, 730 Autumn 1997
... Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Ca ...
... Acidites of phosphonium and sulfonium compounds and ylides (for chemistry see later) Enols, enamines and metalloenamines in synthesis Mechanism of acid and base catalyzed enolization, kinetic vs thermodynamic control Detailed mechanism of -substitution of a carbonyl compound (e. g., bromination) Ca ...
234, advanced chemistry ii - East Pennsboro Area School District
... Rate Constant Reaction Rate L:aw Differential Rate L:aw Integrated Rate Law Method of Initial Rates Initial Rate Overall Reaction Order First Order Reaction Integrated First-Order Rate Law Half-Life of a Reaction Integrated second-Order Rate Law Zero-Order Reaction Integrated Zero-Order Rate Law Pse ...
... Rate Constant Reaction Rate L:aw Differential Rate L:aw Integrated Rate Law Method of Initial Rates Initial Rate Overall Reaction Order First Order Reaction Integrated First-Order Rate Law Half-Life of a Reaction Integrated second-Order Rate Law Zero-Order Reaction Integrated Zero-Order Rate Law Pse ...
Chapter 7
... • In general, the preferential formation of one product because the free energy of activation is lower than another product, therefore, the rate of its formation is faster, is called Kinetic Control of Product Formation. ...
... • In general, the preferential formation of one product because the free energy of activation is lower than another product, therefore, the rate of its formation is faster, is called Kinetic Control of Product Formation. ...
organic chemistry ii
... exhaustive methylation: strategy to determine ring size glycoside formation ...
... exhaustive methylation: strategy to determine ring size glycoside formation ...
File
... At 150C the decomposition of acetaldehyde CH3CHO to methane is a first order reaction. If the rate constant for the reaction at 150C is 0.029 min-1, how long does it take a concentration of 0.050 mol L-1 of acetaldehyde to reduce to a concentration of 0.040 mol L-1? ...
... At 150C the decomposition of acetaldehyde CH3CHO to methane is a first order reaction. If the rate constant for the reaction at 150C is 0.029 min-1, how long does it take a concentration of 0.050 mol L-1 of acetaldehyde to reduce to a concentration of 0.040 mol L-1? ...
4.5: Bonding in Alcohols and Alkyl Halides
... If an electron pair moves in on a new atom, another electron pair must leave so that the atom does not exceed a full valance of eight electrons. There are two common exceptions: A. B. ...
... If an electron pair moves in on a new atom, another electron pair must leave so that the atom does not exceed a full valance of eight electrons. There are two common exceptions: A. B. ...
Examination 1 - Idaho State University
... stoichiometrically until Qc = Ksp. You should be able to decide if, what ppt., and how much ppt. will form if solution which is a mixture of salts. Of course you will need to know the solubility rules to do this. How does adding an acid affect the solubility of certain insoluble ionic compounds? Wha ...
... stoichiometrically until Qc = Ksp. You should be able to decide if, what ppt., and how much ppt. will form if solution which is a mixture of salts. Of course you will need to know the solubility rules to do this. How does adding an acid affect the solubility of certain insoluble ionic compounds? Wha ...
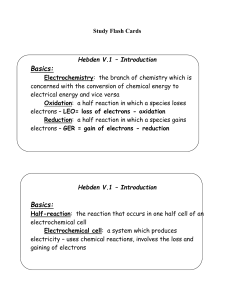
Hebden V.2 – Oxidation Numbers
... List all the entities in the reaction Classify them as RA, OA, or both Find the SOA and SRA – use the table Choose the SOA and write the reduction half reaction Choose the SRA and write the oxidation half reaction Balance the number of electrons lost and gained in the half reaction equations by mult ...
... List all the entities in the reaction Classify them as RA, OA, or both Find the SOA and SRA – use the table Choose the SOA and write the reduction half reaction Choose the SRA and write the oxidation half reaction Balance the number of electrons lost and gained in the half reaction equations by mult ...
Week 6 Solutions - Brown University Wiki
... We know that the oxygen has to make a bond to form a quaternary center. The likely candidate for this is carbon number 4 as it already has three bonds to carbon. If the alcohol oxygen made a bond with carbon 4, it would form the ring system in the product. But how do we make a bond with carbon 4? So ...
... We know that the oxygen has to make a bond to form a quaternary center. The likely candidate for this is carbon number 4 as it already has three bonds to carbon. If the alcohol oxygen made a bond with carbon 4, it would form the ring system in the product. But how do we make a bond with carbon 4? So ...
Lectures 11-12 - U of L Class Index
... properties. Even organic chemists. The alternative is valence bond theory. In valence bond theory, the orbitals on each atom are first “hybridized” so that they can then be combined to make localized bonds. This is more computationally difficult than molecular orbital theory – particularly for lar ...
... properties. Even organic chemists. The alternative is valence bond theory. In valence bond theory, the orbitals on each atom are first “hybridized” so that they can then be combined to make localized bonds. This is more computationally difficult than molecular orbital theory – particularly for lar ...
Lectures 11-12
... properties. Even organic chemists. The alternative is valence bond theory. In valence bond theory, the orbitals on each atom are first “hybridized” so that they can then be combined to make localized bonds. This is more computationally difficult than molecular orbital theory – particularly for lar ...
... properties. Even organic chemists. The alternative is valence bond theory. In valence bond theory, the orbitals on each atom are first “hybridized” so that they can then be combined to make localized bonds. This is more computationally difficult than molecular orbital theory – particularly for lar ...
- M E S KVM College Valanchery.
... The metal centre must normally be able to accommodate both a 16 or 18 valence electron count The metal centre must be able to tolerate more than one ligand geometry The metal centre must be able to undergo oxidation and reduction reactions The catalyst must be contain a third row d-block metal ...
... The metal centre must normally be able to accommodate both a 16 or 18 valence electron count The metal centre must be able to tolerate more than one ligand geometry The metal centre must be able to undergo oxidation and reduction reactions The catalyst must be contain a third row d-block metal ...
Thermodynamics Free-Response
... The reaction is endothermic (∆H = +18 kJ mol-1); an increase in temperature shifts the reaction to favor more products relative to the reactants, resulting in an increase in the value of Keq. d. Both reaction X and reaction Y have solid iodine as a reactant, but the second reactant in reaction X is ...
... The reaction is endothermic (∆H = +18 kJ mol-1); an increase in temperature shifts the reaction to favor more products relative to the reactants, resulting in an increase in the value of Keq. d. Both reaction X and reaction Y have solid iodine as a reactant, but the second reactant in reaction X is ...
Holt Modern Chemistry -
... o chemical equilibrium -- a state of balance in which the rate of a forward reaction equals the rate of the reverse reaction and the concentrations of products and reactants remain unchanged o equilibrium constant -- a number that relates the concentrations of starting materials and products of a re ...
... o chemical equilibrium -- a state of balance in which the rate of a forward reaction equals the rate of the reverse reaction and the concentrations of products and reactants remain unchanged o equilibrium constant -- a number that relates the concentrations of starting materials and products of a re ...
lewis dot structures
... Would occupy these orbitals. If we wanted to write this electron configuration it would be written as follows: ...
... Would occupy these orbitals. If we wanted to write this electron configuration it would be written as follows: ...
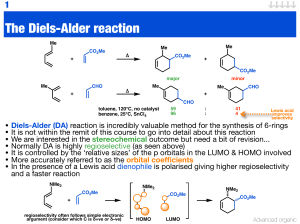
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.