* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download lewis dot structures
Molecular Hamiltonian wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Homoaromaticity wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Marcus theory wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Molecular orbital wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Reflection high-energy electron diffraction wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Degenerate matter wikipedia , lookup
Photoelectric effect wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Chemical bond wikipedia , lookup
Heat transfer physics wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Electron scattering wikipedia , lookup
Atomic theory wikipedia , lookup
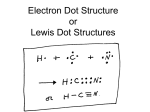
Mr. Shields Regents Chemistry U06 L04 1 Quantum Mechanical Atom Let’s review the orbital structure of the Quantum Mechanical model of the atom: Quantum no. No. of Sublevels Sublevels No. of orbitals 1 1 s 1 2 2 s, p 1, 3 = 4 3 3 s, p, d 1, 3, 5 = 9 4 4 s, p, d, f 1, 3, 5, 7 = 16 5 4 s, p, d, f 1, 3, 5, 7 = 16 6 4 s, p, d, f 1, 3, 5, 7 = 16 7 4 s, p, d, f 1, 3, 5, 7 = 16 2 Energy levels As “n” increases Energy increases n=1 2 3 4 5 6 7 E As sublevels progress from s to p to d to f Energy also increases s p d f E 3 Electron Orbital filling This increasing energy sequence defines Into which Orbitals electrons go as they are added to the atom . The lowest energy levels fill first according To certain specific rules. Remember Rubidium that we looked at earlier? 4 Sublevel Energy increases From s to f. Some sublevels With a lower n may Actually be at a Higher energy level Than some ein a higher n ! 4f > 5s,5p,6s 4d > 5s 3d > 4s 5 Orbital Filling Rules Rule 1: The Aufbau Principle Each electron occupies the lowest energy orbital first - all orbitals in the same energy sublevel are of equal energy - ex: electrons in 2px, 2py, 2pz al have the same energy 6 Orbital Filling Rules Rule 2: The Pauli exclusion Principle Each atomic orbital can only hold a max of 2 electrons - ex: the three p orbitals can each hold 2 electrons so the p sublevel has 6 electrons How many electrons are in the s, d and f sublevels? 7 Orbital Filling Rules Rule 3: Hund’s rule If there is more than 1 sublevel orbital each is filled with One electron before a second is added to each until The sublevel orbitals are filled. - ex: 2px 2py 2pz 8 Electron nomenclature Look at Carbon. It’s atomic # is 6 so it has 6 electrons. According to the rules of orbital filling, it’s electrons Would occupy these orbitals. If we wanted to write this electron configuration it would be written as follows: 1s2 2s2 2p2 2-4 Atomic No. 6 Or we could condense this further and write “2-4” as it is 9 Written in your reference tables. How would we write the electron figuration For these two elements? Atomic # = 7 1s2 2s2 or 2-5 2p3 Atomic # = 8 1s2 2s2 2p4 or 2-6 10 Electronic structure of the Excited State Atomic # = 7 Atomic # = 8 What are the ground states? 1s2 2s2 2p3 1s2 2s2 2p4 One possible excited state (out of many) might be 1s2 2s1 2p33s1 1s1 2s2 2p5 Can you think of other possible excited states? 11 Kernal Nomenclature Look at the electron configuation of Chlorine ( 2-8-7 ) and Neon ( 2-8 ). In the Kernal, or Noble gas, format for designating electron config we write the electron configuration of the atom by: 1) Writing the symbol of the PRECEDING noble gas element in brackets 1) After skipping the electron configuration portion common to both the nobel gas and the atom in question write out the electron configuration of the residual electrons. These should be the outermost principle energy level electrons. 12 Kernal Nomenclature For example chlorine would be represented as [Ne] 3s23p5 Or [Ne] 7. [Ne] designates the 2-8 portion of the electron structure of Chlorine (2-8-7). What’s the noble gas (i.e Kernal) configuration for C? [He] 2s22p2 or simply (Quantum desc.) [He] 4 (Bohr desc.) What is the kernal (noble gas) configuration for the following (use both formats (Quantum and Bohr): K P 13 Valence Electrons Electrons in the outermost principal energy levels are called VALENCE ELECTRONS For the representative elements the valance electrons represent the s and/or p orbitals How many valence electrons do the following atoms have? Li 1 F 7 Ne 8 and what about these… Na 1 Cl 7 Ar 8 notice anything peculiar? 14 Look at the 1st three periods of the periodic table As we move from left to right we add electrons one at a time Valence shell 1e 2e 3e 4e 5e 6e 7e 8e Electrons in the outermost Principal energy level are known as – Valence electrons; the orbit they’re in is known as the valence 15 shell Valence Electrons What do you notice about the number of Electrons in the valence shell of the Noble Gas elements? (i.e. He Ne Ar Kr Xe Rn) Their outermost shell is full and accept for helium they all have 8 electrons. Why doesn’t helium have 8 electrons? 16 The Octet Rule The electron configuration of 8 electrons in The outermost principle energy level is A VERY STABLE electron configuration. It’s know as the OCTET RULE Atoms strive to attain this electron configuration (ns2 np6) by gaining or losing electrons 17 The Octet Rule For example the electron configuration for Sodium is: 1s2 2s2 2p6 3s1 2-8-1 If sodium loses one electron to become an Ion (Na -1e Na+) it’s remaining electrons have the Electron config of Ne (2-8). It’s outermost Energy level now has an octet! 18 Same electron Config as Ne Sodium 19 The Octet Rule Let’s next look at the electron config of Cl 1s2 2s2 2p6 3s2 3p5 or 2-8-7 If Cl gains one electron to become an ion (Cl +1e Cl-) the electron configuration Will have the Electron config of Ar (2-8-8). 20 Lewis Dot Structures Valence electrons are very important In Chemical compound formation so it helps if we can indicate these electrons Schematically These drawings are called LEWIS DOT STRUCTURES. They are created by placing The valence electrons around the elements Symbol. 21 Lewis Dot Structures It easy if you think of an imaginary box surrounding the symbol N Valence Electrons are added as dots at the Center-points of the box lines, one at a time Filling the s Orbital first. These electrons are the atom’s valance Electrons, specifically the s & p electrons. What is the max no. of electrons possible? 8 Ne Mg N 22 Lewis dot structures of Ions Atoms gain or lose electrons to try to Achieve the octet structure. They then become ions. Na -1e Na + (a cation) Br +1e Br - (an anion) In removing an electron From sodium we empty the valence shell and when we add An electron to Bromine we fill the valence shell. In both cases, what is left is the OCTET 23 Lewis dot structures of Ions Lewis Dot structures for ions look the same as they do for Atoms except brackets are used: [ Na ]+ or just Na+ Notice there are no electrons shown as dots. That’s because Na has emptied it’s Valence shell On the other hand, Br filled it’s valence shell. It now Has 8 electons. It’s Lewis dot structure shows these electrons: [ : Br : ] 24 Write the Lewis dot structures for: Al, Al+3, P, I-, S and S-2 25