HL ISSN: 2231 – 3087(print) / 2230 – 9632 (Online)
... room temperature. The Mannich base was then acetylated by refluxing it with acetic anhydride for about 24 hrs and the volatile material was distilled out under reduced pressure to give crude diacetate. It is not purified further and directly treated with conc. Hydrochloric acid to gives 2hydroxy-5-f ...
... room temperature. The Mannich base was then acetylated by refluxing it with acetic anhydride for about 24 hrs and the volatile material was distilled out under reduced pressure to give crude diacetate. It is not purified further and directly treated with conc. Hydrochloric acid to gives 2hydroxy-5-f ...
Elimination Reactions
... In addition to substitution, alkyl halides can also undergo elimination reactions, which lead to the formation of alkenes. As with substitution reactions, elimination reactions come in two mechanistic types: E1 eliminations (a two-step process involving an intermediate carbocation) E2 eliminations ( ...
... In addition to substitution, alkyl halides can also undergo elimination reactions, which lead to the formation of alkenes. As with substitution reactions, elimination reactions come in two mechanistic types: E1 eliminations (a two-step process involving an intermediate carbocation) E2 eliminations ( ...
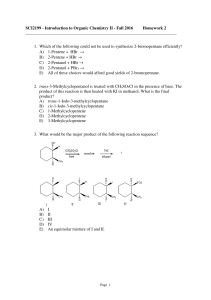
SCI2199 - Introduction to Organic Chemistry II
... SCI2199 - Introduction to Organic Chemistry II - Fall 2016 Homework 2 _______________________________________________________________________ 1. Which of the following could not be used to synthesize 2-bromopentane efficiently? A) 1-Pentene + HBr → B) 2-Pentene + HBr → C) 2-Pentanol + HBr → D) 2-Pen ...
... SCI2199 - Introduction to Organic Chemistry II - Fall 2016 Homework 2 _______________________________________________________________________ 1. Which of the following could not be used to synthesize 2-bromopentane efficiently? A) 1-Pentene + HBr → B) 2-Pentene + HBr → C) 2-Pentanol + HBr → D) 2-Pen ...
Chapter 4: Chemical Reaction Dynamics
... Potential energy profile along the reaction coordinate for H + H2 for different values of the approach angle γ. ...
... Potential energy profile along the reaction coordinate for H + H2 for different values of the approach angle γ. ...
Mechanism
... nitroalkenes. Therefore, only small amounts of base should be used if the isolation of the βhydroxy nitro-compounds is desired. Mechanism of the Henry Reaction The Henry reaction begins with the deprotonation of the nitroalkane on the α-carbon position forming a resonance stabilized anion. This is f ...
... nitroalkenes. Therefore, only small amounts of base should be used if the isolation of the βhydroxy nitro-compounds is desired. Mechanism of the Henry Reaction The Henry reaction begins with the deprotonation of the nitroalkane on the α-carbon position forming a resonance stabilized anion. This is f ...
ap chemistry syllabus - West Essex High School
... ii. Missed homework should be made-up for understanding of concepts d. Attendance i. Follow all rules as stated in student handbook ii. One day to make up work for every day absent iii. Work assigned prior to absence(s) will be due on the first day back e. Academic Integrity i. Students are to hand- ...
... ii. Missed homework should be made-up for understanding of concepts d. Attendance i. Follow all rules as stated in student handbook ii. One day to make up work for every day absent iii. Work assigned prior to absence(s) will be due on the first day back e. Academic Integrity i. Students are to hand- ...
Physical Organic Chemistry
... Another example; the substitution reaction of chlorine by hydroxyl. The reaction temperature decrease when number of EWG increase ...
... Another example; the substitution reaction of chlorine by hydroxyl. The reaction temperature decrease when number of EWG increase ...
Unit 5 Notes
... If you are only given two species (or ions) rather than the four needed for two complete half-reactions as given above, you will need to be able to predict if that particular redox reaction will occur. ...
... If you are only given two species (or ions) rather than the four needed for two complete half-reactions as given above, you will need to be able to predict if that particular redox reaction will occur. ...
heptane
... phantom(a))&&(phantom(a))&& &(H phantom(W))&&&C&(H_3)end(array)... Part A The name of the molecule shown is ...
... phantom(a))&&(phantom(a))&& &(H phantom(W))&&&C&(H_3)end(array)... Part A The name of the molecule shown is ...
Covalent Bonding What is covalent bonding? Hybrid Orbital Formation
... Molecular Orbitals • Lewis structures: accounting for bonding and lone-pair electrons (where are the electrons?) • VSEPR: Electron-pair structure, spatial distribution of electrons (3D) How are bonds made? We know electron distribution in atoms: atomic orbitals: (s, p, d …) What is the electron dis ...
... Molecular Orbitals • Lewis structures: accounting for bonding and lone-pair electrons (where are the electrons?) • VSEPR: Electron-pair structure, spatial distribution of electrons (3D) How are bonds made? We know electron distribution in atoms: atomic orbitals: (s, p, d …) What is the electron dis ...
Name: 1) In a chemical reaction, the difference between the
... Given the system at equilibrium: N2 O4 (g) + 58.1 kJ ‰Š‹ 2NO2 (g) What will be the result of an increase in temperature at constant pressure? A) The equilibrium will shift to the left, and the concentration of NO2 (g) will decrease. B) The equilibrium will shift to the left, and the concentration of ...
... Given the system at equilibrium: N2 O4 (g) + 58.1 kJ ‰Š‹ 2NO2 (g) What will be the result of an increase in temperature at constant pressure? A) The equilibrium will shift to the left, and the concentration of NO2 (g) will decrease. B) The equilibrium will shift to the left, and the concentration of ...
Document
... Alternative possibilities are a head-to-head, tail-to tail configuration _ _ _ _ _ _ _ _ CH2 CH CH CH2 CH2 CH CH X ...
... Alternative possibilities are a head-to-head, tail-to tail configuration _ _ _ _ _ _ _ _ CH2 CH CH CH2 CH2 CH CH X ...
Organic Chemistry Fifth Edition
... An unstable intermediate reacts to form stable products very exothermic. A very favorable process so the activation energy is low. ...
... An unstable intermediate reacts to form stable products very exothermic. A very favorable process so the activation energy is low. ...
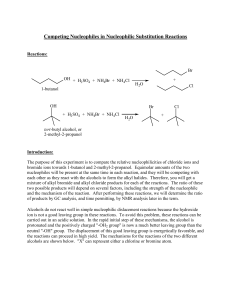
Competing Nucleophiles in Nucleophilic Substitution Reactions
... The sulfuric acid, ammonium bromide, and ammonium chloride will be provided to you as a solvent-nucleophile medium. One mL of this solution contains 0.42 mL of sulfuric acid, 0.1056 g of ammonium chloride, and 0.1944 g of ammonium bromide. From this information, you will be able to calculate the act ...
... The sulfuric acid, ammonium bromide, and ammonium chloride will be provided to you as a solvent-nucleophile medium. One mL of this solution contains 0.42 mL of sulfuric acid, 0.1056 g of ammonium chloride, and 0.1944 g of ammonium bromide. From this information, you will be able to calculate the act ...
pp. 18-21
... What is the formula for ammonium nitrate? for ammonium sulfate? (Do you know the formula and charge of ammonium ion, nitrate ion and sulfate ion?) Once you know the formulas, can you do this WITHOUT a calculator? ...
... What is the formula for ammonium nitrate? for ammonium sulfate? (Do you know the formula and charge of ammonium ion, nitrate ion and sulfate ion?) Once you know the formulas, can you do this WITHOUT a calculator? ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.