* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Covalent Bonding What is covalent bonding? Hybrid Orbital Formation
Survey
Document related concepts
Transcript
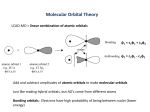
Covalent Bonding What is covalent bonding? Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals Hybrid Orbital Formation Shapes of Hybrid Orbitals Hybrid orbitals and Multiple Bonds resonance structures Mary J. Bojan Chem 110 1 Molecular Orbitals • Lewis structures: accounting for bonding and lone-pair electrons (where are the electrons?) • VSEPR: Electron-pair structure, spatial distribution of electrons (3D) How are bonds made? We know electron distribution in atoms: atomic orbitals: (s, p, d …) What is the electron distribution in molecules? Two models: Valence Bond Theory Valence orbitals on one atom overlap with valence orbitals on another atom: this overlap of orbitals is a covalent bond. Molecular Orbital Theory Covered in Chem 112 Mary J. Bojan Chem 110 2 Covalent Bonding H + H → H2 H (1s) H (1s) H2 molecule Covalent bonding: Mary J. Bojan Chem 110 3 H + H → H2 Two forces operating: • • balance of forces bond length (0.74 Å for H2) Mary J. Bojan Chem 110 4 Bond Types σ-bond • results from • electron density is Examples: s-s s-p pp π-bond • • • results from electron density is Two p-orbitals Mary J. Bojan Chem 110 5 Bonding in CH4 Carbon ground-state: (1s2)2s22p2 1s of H 2p of C Using only unpaired subshell electrons: Expect: The molecule would not have an octet on carbon. Mary J. Bojan Chem 110 6 Orbital Hybridization 1. Promote electrons on C 1s 2. 2s 2p 1s 2s 2p hybridization Four atomic orbitals (2s + 3 × 2p) mix to form four hybrid orbitals (4 × sp3) shake well 1s 3. 2s 2p 1s sp3 Bond formation: _______________________ Form 4 C⎯H bonds by overlapping each hybrid sp3 orbital with an 1s orbital of hydrogen. σ-bond formation One of the four bonds formed by overlap of an sp3 orbital with a hydrogen 1s orbital The new bonds are 109o apart. Mary J. Bojan Chem 110 7 Orbital Hybridization NOTE: start with four atomic orbitals s px py pz end up with four hybrid orbitals 4 sp3 The notation means that each hybrid is composed of 1/4 s and 3/4 p orbitals. Hybrid orbitals: combinations of atomic orbitals (on one atom). better for bonding (more directed) Mary J. Bojan Chem 110 8 sp3 Hybrid Orbitals 1xs+3xp = 4 x sp3 Four atomic orbitals mix to form four hybrid orbitals Mary J. Bojan Chem 110 9 sp and sp2 Hybrid Orbitals 1xs+1xp 2 x sp Two atomic orbitals mix to form two hybrid orbitals 1xs+2xp 3 x sp2 Three atomic orbitals mix to form three hybrid orbitals Mary J. Bojan Chem 110 10 Summary Problem: Can’t use atomic orbitals to describe bonding in molecules Solution: make molecular orbitals by mixing atomic orbitals (call them hybrid orbitals) Two atomic orbitals mix to form two hybrid orbitals 1xs+1xp 2 x sp Three atomic orbitals mix to form three hybrid orbitals 1xs+2xp 3 x sp2 Four atomic orbitals mix to form four hybrid orbitals 1xs+3xp 4 x sp3 Five atomic orbitals mix to form five hybrid orbitals 1 x s + 3 x p +1 x d 5 x sp3d Six atomic orbitals mix to form six hybrid orbitals 1 x s + 3 x p +2 x d 6 x sp3d2 Each hybrid orbital can accommodate 1 pair of electrons. Use VSEPR to determine shape of hybrid orbitals: the electron pairs will get as far from each other as possible. Mary J. Bojan Chem 110 11 Summary of hybridization types The hybridization scheme can be deduced from the electron-pair geometry of the molecule. Number of electron pairs Atomic orbitals used Hybrid type formed 2 s, p two sp linear 3 s, p, p three sp2 trigonal planar 4 s, p, p, p four sp3 tetrahedral CH4, NH3, H2O, NH4+ 5 s, p, p, p, d five sp3d trigonal bipyramidal PF5, SF4, BrF3 6 s, p, p, p, d, d six sp3d2 octahedral SF6, ClF5, XeF4, PF6− Mary J. Bojan Chem 110 Electron-pair geometry Examples BeF2, HgCl2 BF3, SO3, CO32− 12 Multiple Bonds H ethylene: shape about C: hybrid orbitals on C _____ bond angles _______ H C C H H One s and two p atomic orbitals combine to form 3 sp2 hybrid orbitals. One C⎯C and two C⎯H bonds (on each carbon) are formed using sp2 orbitals on carbons. ( σ- bonds) Mary J. Bojan Chem 110 13 Multiple bonds These p-orbitals can overlap, sideways: π-bond Mary J. Bojan Chem 110 14 Orbital Theory of Bonding explains: • Why rotation about double bond does not occur • Why double bonds occur frequently with C, N, and O but not with larger molecules Mary J. Bojan Chem 110 15 Delocalized Orbitals e- pair geometry: hybrid orbitals on N and O are N and O have singly occupied p-orbitals Difference between localized and delocalized π bonding Delocalized bonding brings added stability to a molecule. Molecules with resonance structures have delocalized π bonding Mary J. Bojan Chem 110 16 Reactivity of Hydrocarbons Same reaction: hydrocarbon + Colorless Br2 red ALKANE Heptane + Br2 ⎯→ ALKENE (and ALKYNES) 2-pentene + Br2 ⎯→ AROMATIC toluene + Br2 ⎯→ CH3 Mary J. Bojan Chem 110 17 Stability of aromatic hydrocarbons Alkene + Br2 reacts readily Aromatic + Br2: no reaction • π bonds of alkenes are very reactive toward addition. (π bonds in alkynes even more so.) • π-bonds in benzene are NOT reactive due to the extra stability of delocalized π system Mary J. Bojan Chem 110 18 Summary of Covalent bonding 1. Draw Lewis Structure 2. Use VSEPR to determine shape e- pair geometry molecular geometry 3. What hybrid orbitals are involved in bonding? Determined by electron pair geometry. (Know the shapes of the hybrid orbitals.) 4. Is the molecule polar? Determined by molecular geometry. Remember: Each single bond = covalent bond = σ bond A covalent bond forms when orbitals overlap. σ-bond: head-on overlap π-bond: sidewise overlap Mary J. Bojan Chem 110 19 Determine the hybrid orbitals on the nitrogen in angle 1 and the C in angle 2. A. B. C. D. E. Mary J. Bojan Angle #1 sp3 sp sp2 sp2 sp3 Angle #2 sp2 sp2 sp3 sp sp3 Chem 110 20