Polarity of covalent bonds
... the oxygen atom has two bonds with H and two lone electron pairs (as can be seen with the valence bond theory as well from the electronic configuration of oxygen), which means there are four such 'elements' on O. The model molecule is, then, AX4: sp3 hybridisation is utilized, and the electron arran ...
... the oxygen atom has two bonds with H and two lone electron pairs (as can be seen with the valence bond theory as well from the electronic configuration of oxygen), which means there are four such 'elements' on O. The model molecule is, then, AX4: sp3 hybridisation is utilized, and the electron arran ...
Addition Reactions
... acid-catalyzed hydration of an alkene is regioselective; hydrogen adds preferentially to the sp2 carbon with less # of hydrogens. ...
... acid-catalyzed hydration of an alkene is regioselective; hydrogen adds preferentially to the sp2 carbon with less # of hydrogens. ...
Organic Chemistry - Snow College | It's SNOWing
... a more electronegative atom. What happens when it is connected to a less electronegative atom? ...
... a more electronegative atom. What happens when it is connected to a less electronegative atom? ...
Reaction Rate review questions
... 2. The reactant particles must collide hard enough to break old chemical bonds so that new chemical bonds will form. Bond breaking is an endothermic process. The minimum energy that colliding particles must have in order to react is called the activation energy. 3. The colliding reactants particles ...
... 2. The reactant particles must collide hard enough to break old chemical bonds so that new chemical bonds will form. Bond breaking is an endothermic process. The minimum energy that colliding particles must have in order to react is called the activation energy. 3. The colliding reactants particles ...
Chapter 1 Heterogeneous catalysis - diss.fu
... poisoning, fouling and sintering [5]. Consequently, a fundamental understanding of heterogeneous catalytic reactions at a molecular level faces the added complexities of the reaction mechanism, the catalyst surface, composition and morphology and the different timescales at which relevant phenomena ...
... poisoning, fouling and sintering [5]. Consequently, a fundamental understanding of heterogeneous catalytic reactions at a molecular level faces the added complexities of the reaction mechanism, the catalyst surface, composition and morphology and the different timescales at which relevant phenomena ...
Hybridization and St..
... Hybridization also occurs in compounds of beryllium. The electron configuration if Be is 1s22s2. It would appear to have no half-filled orbitals with which to form covalent bonds. ...
... Hybridization also occurs in compounds of beryllium. The electron configuration if Be is 1s22s2. It would appear to have no half-filled orbitals with which to form covalent bonds. ...
Untitled - Menihek Home Page
... He said when it is disturbed, the equilibrium will shift to try and compensate for the change. This will cause the concentrations of reactants and products to change until a new equilibrium forms. Either the forward reaction rate will increase, which decreases the concentrations of the reactants and ...
... He said when it is disturbed, the equilibrium will shift to try and compensate for the change. This will cause the concentrations of reactants and products to change until a new equilibrium forms. Either the forward reaction rate will increase, which decreases the concentrations of the reactants and ...
Organic Chemistry I Laboratory
... were investigating a chemical reaction both backward and forward. Markovnikov was adding hydrogen iodide to alkenes to prepare alkyl iodides, and Zaitzev was removing hydrogen iodide from alkyl iodides to prepare alkenes. Markovnikov discovered that hydrogen iodide adds to propene to form mainly 2-i ...
... were investigating a chemical reaction both backward and forward. Markovnikov was adding hydrogen iodide to alkenes to prepare alkyl iodides, and Zaitzev was removing hydrogen iodide from alkyl iodides to prepare alkenes. Markovnikov discovered that hydrogen iodide adds to propene to form mainly 2-i ...
CHM 101 THERMOCHEMISTRY DEFINITIONS ENERGY is the
... negative value of ΔU indicates that the system as lost energy to its surroundings. ΔU = Q + W, Q is the heat absorbed or evolved by the system. U increases when work is done on a system or heat is added to a system. Sign conventions for Q, W and ΔU +Q = the system gains heat —Q = the system losses h ...
... negative value of ΔU indicates that the system as lost energy to its surroundings. ΔU = Q + W, Q is the heat absorbed or evolved by the system. U increases when work is done on a system or heat is added to a system. Sign conventions for Q, W and ΔU +Q = the system gains heat —Q = the system losses h ...
POGIL.CH7B.Tro
... 8. What is the relationship between the value ofthe azimuthal quantum number and the nmnber l of angular nodal planes? _ 9. In addition to angular nodal planes, there are radial nodes. These nodes occur for all angles at some distance from the nucleus. Which orbitals have radial nodes? 10. For a giv ...
... 8. What is the relationship between the value ofthe azimuthal quantum number and the nmnber l of angular nodal planes? _ 9. In addition to angular nodal planes, there are radial nodes. These nodes occur for all angles at some distance from the nucleus. Which orbitals have radial nodes? 10. For a giv ...
PAKISTAN SHIPOWNERS` GOVERNMENT COLLEGE,
... c) What is Allotropy? Describe different allotropic form of Sulphur. d) Describe the manufacture of Sulphuric acid H2SO4 by Contact process. Draw the flow sheet diagram 5. a) What is metallurgy? Explain the extraction of 99.99% pure aluminium from bauxite ore containing SiO 2 and Fe2O3 as impurities ...
... c) What is Allotropy? Describe different allotropic form of Sulphur. d) Describe the manufacture of Sulphuric acid H2SO4 by Contact process. Draw the flow sheet diagram 5. a) What is metallurgy? Explain the extraction of 99.99% pure aluminium from bauxite ore containing SiO 2 and Fe2O3 as impurities ...
Examlette 1 - Bryn Mawr College
... 7. The standard free energy of formation for N2O4 is +97 kJ/mol and indicates that N2O4 is thermodynamically unstable towards decomposition to the elements. a) Explain what the term “standard free energy of formation “ means. Standard Free Energy refers to the energy required to form a molecule from ...
... 7. The standard free energy of formation for N2O4 is +97 kJ/mol and indicates that N2O4 is thermodynamically unstable towards decomposition to the elements. a) Explain what the term “standard free energy of formation “ means. Standard Free Energy refers to the energy required to form a molecule from ...
Electron domain and molecular geometry of bro2-
... Molecular Structure Calculations.. Molecular Structure Calculations A quick explanation of the molecular geometry of ClO3- including a description of the ClO3- bond angles. Looking at the ClO3- Lewis structure we can see. A quick explanation of the molecular geometry of SO2 including a description o ...
... Molecular Structure Calculations.. Molecular Structure Calculations A quick explanation of the molecular geometry of ClO3- including a description of the ClO3- bond angles. Looking at the ClO3- Lewis structure we can see. A quick explanation of the molecular geometry of SO2 including a description o ...
L-13
... the reaction and led to the production of allylated product 3a in 80% yield (entry 2).[6] Strong Lewis acids such as AlCl3 or BF3・OEt2 were not effective for the allylation (entries 3 and 4), probably because these catalysts are not stable under protic conditions. Sc(OTf)3 only gave a low yield of 3 ...
... the reaction and led to the production of allylated product 3a in 80% yield (entry 2).[6] Strong Lewis acids such as AlCl3 or BF3・OEt2 were not effective for the allylation (entries 3 and 4), probably because these catalysts are not stable under protic conditions. Sc(OTf)3 only gave a low yield of 3 ...
Lecture Resource ()
... Organometallic Compounds An organic compound containing a carbon–metal bond ...
... Organometallic Compounds An organic compound containing a carbon–metal bond ...
haloalkanes - Knockhardy
... Complication The OH¯ removes a proton from a carbon atom adjacent the C bearing the halogen. If there had been another carbon atom on the other side of the C-Halogen bond, its hydrogen(s) would also be open to attack. If the haloalkane is unsymmetrical (e.g. 2-bromobutane) a mixture of isomeric alke ...
... Complication The OH¯ removes a proton from a carbon atom adjacent the C bearing the halogen. If there had been another carbon atom on the other side of the C-Halogen bond, its hydrogen(s) would also be open to attack. If the haloalkane is unsymmetrical (e.g. 2-bromobutane) a mixture of isomeric alke ...
esters - wellswaysciences
... formed in the reaction the hydroxide ions present react with them to form a salt. • This removes the acid from the reaction mixture and so the reaction moves RIGHT. • The base (or alkali) is used up in the reaction. • This is not strictly catalysed by the alkali. Why not? ...
... formed in the reaction the hydroxide ions present react with them to form a salt. • This removes the acid from the reaction mixture and so the reaction moves RIGHT. • The base (or alkali) is used up in the reaction. • This is not strictly catalysed by the alkali. Why not? ...
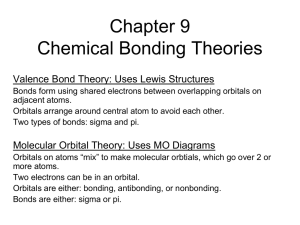
Chapter 10 Chemical Bonding Theories
... adjacent atoms. Orbitals arrange around central atom to avoid each other. Two types of bonds: sigma and pi. ...
... adjacent atoms. Orbitals arrange around central atom to avoid each other. Two types of bonds: sigma and pi. ...
Woodward–Hoffmann rules

The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules in organic chemistry predicting the barrier heights of pericyclic reactions based upon conservation of orbital symmetry. The Woodward–Hoffmann rules can be applied to understand electrocyclic reactions, cycloadditions (including cheletropic reactions), sigmatropic reactions, and group transfer reactions. Reactions are classified as allowed if the electronic barrier is low, and forbidden if the barrier is high. Forbidden reactions can still take place but require significantly more energy.The Woodward–Hoffmann rules were first formulated to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. Thermolysis of the substituted cyclobutene trans-1,2,3,4-tetramethylcyclobutene (1) gave only one diastereomer, the (E,E)-3,4-dimethyl-2,4-hexadiene (2) as shown below; the (Z,Z) and the (E,Z) diastereomers were not detected in the reaction. Similarly, thermolysis of cis-1,2,3,4-tetramethylcyclobutene (3) gave only the (E,Z) diastereomer (4).Due to their elegance and simplicity, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for this work, shared with Kenichi Fukui who developed a similar model using frontier molecular orbital (FMO) theory; because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.