Template for calculating the ΔH° in a multiple step chemical reaction
... To make Al2O3, you need the O3 from Fe2O3. To do that, you must first carry out the process of breaking up the Fe2O3, which has its own enthalpy of formation. However we are not forming Fe2O3. We are decomposing it. So, we must reverse the equation. Fe2O3 → 2Fe(s) + 3/2 O2 (g) Since the equation is ...
... To make Al2O3, you need the O3 from Fe2O3. To do that, you must first carry out the process of breaking up the Fe2O3, which has its own enthalpy of formation. However we are not forming Fe2O3. We are decomposing it. So, we must reverse the equation. Fe2O3 → 2Fe(s) + 3/2 O2 (g) Since the equation is ...
Chem 341 Review for Finals Key Reactions Mechanisms
... - Sn1, Sn2, E2 - Carbocation stabilities ...
... - Sn1, Sn2, E2 - Carbocation stabilities ...
fulltext $(function(){PrimeFaces.cw("Tooltip","widget_formSmash_items_resultList_20_j_idt799_0_j_idt801",{id:"formSmash:items:resultList:20:j_idt799:0:j_idt801",widgetVar:"widget_formSmash_items_resultList_20_j_idt799_0_j_idt801",showEffect:"fade",hideEffect:"fade",target:"formSmash:items:resultList:20:j_idt799:0:fullText"});});
... para positions on the aromatic rings as well as 2naphthylmethanol were well-tolerated (Entries 2, 3 and 5). More catalyst (1.0 mol%) was necessary to complete the reaction when (2-methylphenyl)methanol and 1-naphthylmethanol were used as substrates (Entries 4 and 6). Two heteroaromatic alcohols, fur ...
... para positions on the aromatic rings as well as 2naphthylmethanol were well-tolerated (Entries 2, 3 and 5). More catalyst (1.0 mol%) was necessary to complete the reaction when (2-methylphenyl)methanol and 1-naphthylmethanol were used as substrates (Entries 4 and 6). Two heteroaromatic alcohols, fur ...
Carbohydrates important reactions
... When the aldehyde function of an aldose is oxidized to a carboxylic acid the product is called an aldonic acid. Because of the 2º hydroxyl functions that are also present in these compounds, a mild oxidizing agent such as hypobromite must be used for this conversion (equation 1). If both ends of an ...
... When the aldehyde function of an aldose is oxidized to a carboxylic acid the product is called an aldonic acid. Because of the 2º hydroxyl functions that are also present in these compounds, a mild oxidizing agent such as hypobromite must be used for this conversion (equation 1). If both ends of an ...
Alcohols - Calderglen High School
... Peeled apples turn brown due to the reaction of compounds called phenols. The first two steps in the reaction of one phenol, A are; ...
... Peeled apples turn brown due to the reaction of compounds called phenols. The first two steps in the reaction of one phenol, A are; ...
Synthesis of Isobutyl Propionate via Esterification
... Another way to upset the equilibrium is to remove water. This can be done by adding to the reaction mixture molecular sieves, an artificial zeolite, which preferentially adsorb water. Most other drying agents, such as anhydrous sodium sulfate or calcium chloride, will not remove water at the tempera ...
... Another way to upset the equilibrium is to remove water. This can be done by adding to the reaction mixture molecular sieves, an artificial zeolite, which preferentially adsorb water. Most other drying agents, such as anhydrous sodium sulfate or calcium chloride, will not remove water at the tempera ...
Reductive Coupling Reactions of Nitrones and Imines
... N-tert-Butanesulfinyl groups (15) have also served as chiral auxiliaries in the cross coupling of nitrones and imines to generate unsymmetrical 1,2-diamines18 in moderate to good yields with excellent diastereoselectivity. Reduced yields were obtained for sterically bulky substituents, and no reacti ...
... N-tert-Butanesulfinyl groups (15) have also served as chiral auxiliaries in the cross coupling of nitrones and imines to generate unsymmetrical 1,2-diamines18 in moderate to good yields with excellent diastereoselectivity. Reduced yields were obtained for sterically bulky substituents, and no reacti ...
Organic Synthesis Part 2
... Reaction with carbonyl compounds is by donation of hydride, to give a transient alkoxide/alane pair which combines to give an alkoxytrihydroaluminate. This can go on to donate the remaining three hydrides in the same way, although at a reduced rate. We can exploit this by deliberately making trialko ...
... Reaction with carbonyl compounds is by donation of hydride, to give a transient alkoxide/alane pair which combines to give an alkoxytrihydroaluminate. This can go on to donate the remaining three hydrides in the same way, although at a reduced rate. We can exploit this by deliberately making trialko ...
Lesmahagow High School CfE Advanced Higher Chemistry Unit 2
... Propanol which is an alcohol and thus contains the OH group will also display hydrogen bonding. Thinking back the higher course each of the intermolecular attractions will give rise to differences in both the physical and chemical properties of a molecule. Boiling Points—most organic molecules conta ...
... Propanol which is an alcohol and thus contains the OH group will also display hydrogen bonding. Thinking back the higher course each of the intermolecular attractions will give rise to differences in both the physical and chemical properties of a molecule. Boiling Points—most organic molecules conta ...
Chemistry 211 - MiraCosta College
... O. Carboxyl group protection P. Peptide bond formation Q. Solid-phase peptide synthesis, Merrifield method R. Secondary structures of peptides and proteins S. Tertiary structures of peptides and proteins T. Coenzymes U. Protein quarternary structure, hemoglobin V. Pyrimidines and purines W. Nucleosi ...
... O. Carboxyl group protection P. Peptide bond formation Q. Solid-phase peptide synthesis, Merrifield method R. Secondary structures of peptides and proteins S. Tertiary structures of peptides and proteins T. Coenzymes U. Protein quarternary structure, hemoglobin V. Pyrimidines and purines W. Nucleosi ...
Organic Reactions
... ∆G is negative, energy is released, exergonic reaction ∆G is positive, energy absorbed, endergonic reaction ...
... ∆G is negative, energy is released, exergonic reaction ∆G is positive, energy absorbed, endergonic reaction ...
Chapter 18 - Aldehydes and Ketones
... However, it is possible to reduce the reactivity of the hydride reagent by replacing some hydrogen atoms by alkoxy groups. In such cases, the reagent is not active enough to reduce the intermediate, and the aldehyde is obtained. ...
... However, it is possible to reduce the reactivity of the hydride reagent by replacing some hydrogen atoms by alkoxy groups. In such cases, the reagent is not active enough to reduce the intermediate, and the aldehyde is obtained. ...
Chapter 1 Organoaluminum Reagents for Selective Organic
... Chapter 3. Bulky Aluminum Reagents for Selective Organic Synthesis In chapter 2 we discussed several excellent methods of discriminating various functional groups using bulky aluminum reagents. In this section we focus on the reactions promoted with bulky aluminum reagents which could not be achiev ...
... Chapter 3. Bulky Aluminum Reagents for Selective Organic Synthesis In chapter 2 we discussed several excellent methods of discriminating various functional groups using bulky aluminum reagents. In this section we focus on the reactions promoted with bulky aluminum reagents which could not be achiev ...
Macromolecules
... where the starch is broken down into the various monosaccharides. A major product is glucose, which can be used immediately for metabolism to make energy. ...
... where the starch is broken down into the various monosaccharides. A major product is glucose, which can be used immediately for metabolism to make energy. ...
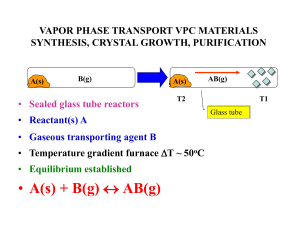
vapor phase transport vpc materials synthesis, crystal growth
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
CN>Chapter 22CT>Carbonyl Alpha
... In the haloform reaction, there is an -substitution whereby the methyl ketone is trihalogenated at the position. The trihalomethyl group is displaced by –OH. This reaction is used as a test for methyl ketones. + reactions would come from reactions a, and b; while – reactions would come from c, d, ...
... In the haloform reaction, there is an -substitution whereby the methyl ketone is trihalogenated at the position. The trihalomethyl group is displaced by –OH. This reaction is used as a test for methyl ketones. + reactions would come from reactions a, and b; while – reactions would come from c, d, ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.