Microsoft Word
... aldehydes with nitroalkane in moderate to excellent yields using nanocrystalline magnesium oxide as a reusable catalyst was developed for the first time. The nitro group of the niroaldol reaction products can undergo the Nef reaction, reduction to amino group, or nucleophilic displacement. The resul ...
... aldehydes with nitroalkane in moderate to excellent yields using nanocrystalline magnesium oxide as a reusable catalyst was developed for the first time. The nitro group of the niroaldol reaction products can undergo the Nef reaction, reduction to amino group, or nucleophilic displacement. The resul ...
102 Lab 7 Esters Fall05
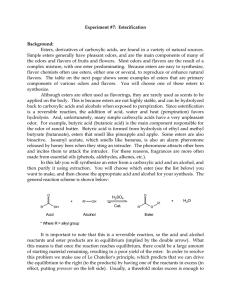
... flavor chemists often use esters, either one or several, to reproduce or enhance natural flavors. The table on the next page shows some examples of esters that are primary components of various odors and flavors. You will choose one of these esters to synthesize. Although esters are often used as fl ...
... flavor chemists often use esters, either one or several, to reproduce or enhance natural flavors. The table on the next page shows some examples of esters that are primary components of various odors and flavors. You will choose one of these esters to synthesize. Although esters are often used as fl ...
File - Dr KHALID SHADID
... This reaction, called the Bacyer-Villiger oxidation, is especially useful with ketnne because it convert them to ...
... This reaction, called the Bacyer-Villiger oxidation, is especially useful with ketnne because it convert them to ...
Catalytic, Enantioselective Alkylations of N,O- and
... acetophenone developed. In addition, the alcohol proton disappeared, indicating Osilylation (Scheme 1). A second equiv of enol silane 4a was then added to the mixture and the reaction was monitored; no product formation was noted even after extended periods of time. After addition of the catalyst 2 ...
... acetophenone developed. In addition, the alcohol proton disappeared, indicating Osilylation (Scheme 1). A second equiv of enol silane 4a was then added to the mixture and the reaction was monitored; no product formation was noted even after extended periods of time. After addition of the catalyst 2 ...
Chemistry Name Mr. Reger Review Guide – Ch. 9
... 10. Using the reaction below, how many grams of NaCl can be produced from 10.9 g NaOH? 3 Cl2(g) + 6 NaOH(aq) 5 NaCl(aq) + NaClO3(aq) + 3 H2O(l) 11. Use the equation in the question above to answer the following: a) What is the theoretical yield of NaClO3 if 4.0mol Cl2 is reacted with excess NaOH? ...
... 10. Using the reaction below, how many grams of NaCl can be produced from 10.9 g NaOH? 3 Cl2(g) + 6 NaOH(aq) 5 NaCl(aq) + NaClO3(aq) + 3 H2O(l) 11. Use the equation in the question above to answer the following: a) What is the theoretical yield of NaClO3 if 4.0mol Cl2 is reacted with excess NaOH? ...
11. Reactions of Alkyl Halides
... delocalization of charge (See Figure 11-13) – Primary allylic and benzylic are also more reactive in the SN2 mechanism ...
... delocalization of charge (See Figure 11-13) – Primary allylic and benzylic are also more reactive in the SN2 mechanism ...
Chem Stoichiometry Study Guide
... 16. SO2(g) 17. Magnesium metal is added to aqueous hydrochloric acid. 18. Potassium metal is combined with chlorine gas. 19. Aqueous solutions of potassium bromide and silver nitrate are combined. ...
... 16. SO2(g) 17. Magnesium metal is added to aqueous hydrochloric acid. 18. Potassium metal is combined with chlorine gas. 19. Aqueous solutions of potassium bromide and silver nitrate are combined. ...
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015
... Formation of the bromonium ion explains stereochemical outcome ...
... Formation of the bromonium ion explains stereochemical outcome ...
Module - EPS School Projects - Heriot
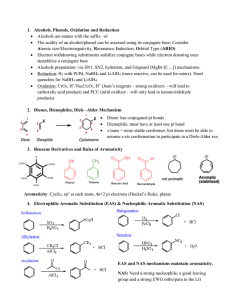
... Addition Reactions – Revision of Markownikoff addition to alkenes, with further examples; mercuric-catalysed hydration of alkenes and alkynes; hydroboration of alkenes and alkynes, including mechanism, stereochemistry, hindered boranes. Reductions in Organic Chemistry – Catalytic reduction, includin ...
... Addition Reactions – Revision of Markownikoff addition to alkenes, with further examples; mercuric-catalysed hydration of alkenes and alkynes; hydroboration of alkenes and alkynes, including mechanism, stereochemistry, hindered boranes. Reductions in Organic Chemistry – Catalytic reduction, includin ...
thiols and sulfides.
... between strain, entropy, and proximity. Entropy reduction (due to ring closure) increases with increasing ring size. (Reaction rate decrease with increasing ring size.) Ring strain decreases with increasing ring size. (Reaction rate increase with increasing ring size.) Transition-state strain is red ...
... between strain, entropy, and proximity. Entropy reduction (due to ring closure) increases with increasing ring size. (Reaction rate decrease with increasing ring size.) Ring strain decreases with increasing ring size. (Reaction rate increase with increasing ring size.) Transition-state strain is red ...
An Efficient Method for Selective Deprotection of Trimethylsilyl
... wish herein to introduce 1-butyl-4-aza-1-azoniabicyclo[2.2.2] octane dichromate 3 for deprotection oxidation of trimethylsilyl ether and tetrahydropyranyl ether to the corresponding carbonyl compounds in the presence of AlCl under solvent-free conditions. This new reagent has been readily prepared b ...
... wish herein to introduce 1-butyl-4-aza-1-azoniabicyclo[2.2.2] octane dichromate 3 for deprotection oxidation of trimethylsilyl ether and tetrahydropyranyl ether to the corresponding carbonyl compounds in the presence of AlCl under solvent-free conditions. This new reagent has been readily prepared b ...
Synthesis of Oil of Wintergreen - Cornell University
... If safety issues are a concern this lab could easily be used as a teacher demonstration ...
... If safety issues are a concern this lab could easily be used as a teacher demonstration ...
Exp`t 73
... Interestingly, distillation of the product mixture gives fractions of varying composition, where the lower boiling product distills later. If all of the products were formed instantaneously, then obviously the lower boiling component would be expected to distill earlier; that it does not implies tha ...
... Interestingly, distillation of the product mixture gives fractions of varying composition, where the lower boiling product distills later. If all of the products were formed instantaneously, then obviously the lower boiling component would be expected to distill earlier; that it does not implies tha ...
A Straightforward Route to Enantiopure Pyrrolizidines and
... large excess of reagent in refluxing chloroform, no oxidation to the sulfonates was observed [26]. Since in a similar case [24] even treatment with excess trifluoroperacetic acid (CH2Cl2, reflux) and other reagents [27] was unsuccessful, we tested potassium permanganate as a strong oxidant. In a fir ...
... large excess of reagent in refluxing chloroform, no oxidation to the sulfonates was observed [26]. Since in a similar case [24] even treatment with excess trifluoroperacetic acid (CH2Cl2, reflux) and other reagents [27] was unsuccessful, we tested potassium permanganate as a strong oxidant. In a fir ...
Active pharmaceutical ingredients and amino acids as building
... obtain a great variety of non-toxic API-ILs based on amino acids. In addition, this one-step procedure is an atom-economic reaction without any poisonous by-product. ...
... obtain a great variety of non-toxic API-ILs based on amino acids. In addition, this one-step procedure is an atom-economic reaction without any poisonous by-product. ...
Chapter 3: Chemical Reactions and the Earth`s Composition
... Example 2: Everclear is a brand of grain alcohol that can be as high as 190 proof (or 95% ethanol, C2H5OH, by volume). Calculate the mass of carbon dioxide produced upon complete combustion of the ethanol in a 750 mL bottle of Everclear. Write the balanced chemical equation for the combustion of eth ...
... Example 2: Everclear is a brand of grain alcohol that can be as high as 190 proof (or 95% ethanol, C2H5OH, by volume). Calculate the mass of carbon dioxide produced upon complete combustion of the ethanol in a 750 mL bottle of Everclear. Write the balanced chemical equation for the combustion of eth ...
Chapter 8 - profpaz.com
... Consequently, unless there are more ingredients, only 15 pancakes can be made from the combination of the above ingredients. This is due to the flour being completely used up after making 15 pancakes. ...
... Consequently, unless there are more ingredients, only 15 pancakes can be made from the combination of the above ingredients. This is due to the flour being completely used up after making 15 pancakes. ...
Enantioselective Henry Reactions under Dual Lewis Acid/Amine
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
Relative Reactivity of Aldehydes and Ketones: Generally
... 1. Reductions of aldehydes and ketones- OLD • Reagents: NaBH4 or LiAlH4 The hydride, H-, is a strong nucleophile and this reaction process is “irreversible”. Strong nucleophiles are typically poor leaving groups (cannot stabilize an anion), thus once the nucleophile has completed its bond formation, ...
... 1. Reductions of aldehydes and ketones- OLD • Reagents: NaBH4 or LiAlH4 The hydride, H-, is a strong nucleophile and this reaction process is “irreversible”. Strong nucleophiles are typically poor leaving groups (cannot stabilize an anion), thus once the nucleophile has completed its bond formation, ...
The Grignard Reagent
... • It is very important that the reaction apparatus, reagents, and solvents must all be kept dry. You will be using glassware from your organic lab kits for this experiment. It is a good idea to dry your glassware in the oven before starting the reaction. Also make sure to keep the reagent bottles ca ...
... • It is very important that the reaction apparatus, reagents, and solvents must all be kept dry. You will be using glassware from your organic lab kits for this experiment. It is a good idea to dry your glassware in the oven before starting the reaction. Also make sure to keep the reagent bottles ca ...
Chem 400 Review Chem 350 JJ.S17
... Electron withdrawing substituents stabilize conjugate bases while electron donating ones destabilize a conjugate base Alcohols preparation: via SN1, SN2, hydration, and Grignard (MgBr-[C…]) mechanisms Reduction: H2 with Pt/Pd, NaBH4 and LiAlH4 (more reactive, can be used for esters). Need quen ...
... Electron withdrawing substituents stabilize conjugate bases while electron donating ones destabilize a conjugate base Alcohols preparation: via SN1, SN2, hydration, and Grignard (MgBr-[C…]) mechanisms Reduction: H2 with Pt/Pd, NaBH4 and LiAlH4 (more reactive, can be used for esters). Need quen ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.