Dehydration notes
... Mass of distillate is X g but only 85.82% is alkene products so… 0.8582 * X g = Y g of mixed alkenes ...
... Mass of distillate is X g but only 85.82% is alkene products so… 0.8582 * X g = Y g of mixed alkenes ...
Remodeling of the natural product fumagillol
... transannular reactions.12 The Miller group from Notre Dame has incorporated oxazine heterocycles into natural products bearing 1,3-butadiene subunits employing iminonitroso Diels-Alder cycloadditions.13,14 Further evolution of these ideas would involve the creation of a diverse library of remodelled ...
... transannular reactions.12 The Miller group from Notre Dame has incorporated oxazine heterocycles into natural products bearing 1,3-butadiene subunits employing iminonitroso Diels-Alder cycloadditions.13,14 Further evolution of these ideas would involve the creation of a diverse library of remodelled ...
Recall
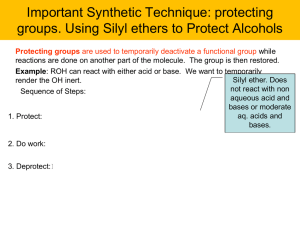
... Protecting groups are used to temporarily deactivate a functional group while reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily Silyl ether. Does render the OH inert. not react with non Sequence of ...
... Protecting groups are used to temporarily deactivate a functional group while reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily Silyl ether. Does render the OH inert. not react with non Sequence of ...
Kazzie`s Guide to Orgo 2
... General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 210 Stuff Identify ...
... General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 210 Stuff Identify ...
Carbonyl Compounds
... addition elimination (condensation) reactions with NH2OH and ,2,4-DNPH . Oxidation (aldehyde can be easily oxidized to RCOOH, but ketone is resistant to oxidation & cannot be easily oxidized) ...
... addition elimination (condensation) reactions with NH2OH and ,2,4-DNPH . Oxidation (aldehyde can be easily oxidized to RCOOH, but ketone is resistant to oxidation & cannot be easily oxidized) ...
Inorganic Reaction Mechanisms at the Molecular Level Oxford
... these types of reactions are not amenable to selective generation of primary oxidation products such as alcohols from alkanes. For these types of oxidation reactions, due to the low homolytic bond strength of the CH bonds of the alcohol product compared to the starting alkane, transition states or i ...
... these types of reactions are not amenable to selective generation of primary oxidation products such as alcohols from alkanes. For these types of oxidation reactions, due to the low homolytic bond strength of the CH bonds of the alcohol product compared to the starting alkane, transition states or i ...
ppt
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
Experiment 7 – Dehydration of Methylcyclohexanols
... product. The regiospecificity of the reaction is dependent on Zaitsev’s rule, where the major product tends to be the more substituted alkene. When two different products are possible, these products are constitutional isomers of each other or in this case can be referred to as regioisomers. The typ ...
... product. The regiospecificity of the reaction is dependent on Zaitsev’s rule, where the major product tends to be the more substituted alkene. When two different products are possible, these products are constitutional isomers of each other or in this case can be referred to as regioisomers. The typ ...
Rapid Microwave Synthesis, Characterization and Reactivity
... parameters of the N and H atoms within both the LT- and HT-Li4NH phases. Simultaneous thermal analysis (thermogravimetric and differential thermal analysis; TG-DTA) was performed using a NETZSCH STA 409PC thermobalance coupled to a HIDEN HPR20 mass spectrometer (MS). Approximately 30 mg of Li4NH was ...
... parameters of the N and H atoms within both the LT- and HT-Li4NH phases. Simultaneous thermal analysis (thermogravimetric and differential thermal analysis; TG-DTA) was performed using a NETZSCH STA 409PC thermobalance coupled to a HIDEN HPR20 mass spectrometer (MS). Approximately 30 mg of Li4NH was ...
Ether And Epoxides
... substitution occurs and formation of alcohols from alkyl halide takes place. Chemical properties (i) Oxidation Ether are less reactive due to absence of polarity, along with an ability to soluble in nonpolar substances like CCl4, that makes ethers so often used as solvents when carrying out many org ...
... substitution occurs and formation of alcohols from alkyl halide takes place. Chemical properties (i) Oxidation Ether are less reactive due to absence of polarity, along with an ability to soluble in nonpolar substances like CCl4, that makes ethers so often used as solvents when carrying out many org ...
Chapter 15 Multistep Syntheses
... Alcohol to alkyl halide with PX3 (x=Br, Cl, I) alkyl halide to alcohol with NaOH Ketone to Ketal with acid/ROH Ketal to Keone with acid/H2O Ester to Acid with NaOH followed by acidification Acid to Ester with acid and ROH Nitrile to Amide with NaOH/H2O Amide to Nitrile with POCl3 Acid chloride to Ac ...
... Alcohol to alkyl halide with PX3 (x=Br, Cl, I) alkyl halide to alcohol with NaOH Ketone to Ketal with acid/ROH Ketal to Keone with acid/H2O Ester to Acid with NaOH followed by acidification Acid to Ester with acid and ROH Nitrile to Amide with NaOH/H2O Amide to Nitrile with POCl3 Acid chloride to Ac ...
Organic Reactions Worksheet
... b. Using a primary alcohol i) Give the displayed (structural formula) which it could be oxidized to ii) Give the conditions needed to get each product iii) State which homologous series the products are part of iv) Write the balanced equation for each oxidizing reaction, use [O] convention c. Using ...
... b. Using a primary alcohol i) Give the displayed (structural formula) which it could be oxidized to ii) Give the conditions needed to get each product iii) State which homologous series the products are part of iv) Write the balanced equation for each oxidizing reaction, use [O] convention c. Using ...
CHM 222 - Jefferson State Community College
... Demonstrate an understanding of reactions involving aldehydes, ketones, carboxylic acids and their derivatives including nomenclature, synthesis and mechanisms. 1. Name and draw aldehydes, ketones, carboxylic acids and their derivatives. 2. Propose a synthesis for each type of compound listed above. ...
... Demonstrate an understanding of reactions involving aldehydes, ketones, carboxylic acids and their derivatives including nomenclature, synthesis and mechanisms. 1. Name and draw aldehydes, ketones, carboxylic acids and their derivatives. 2. Propose a synthesis for each type of compound listed above. ...
The first practical method for asymmetric epoxidation
... small excess (2-5%) to be safe. This consideration is more important for small-scale reactions where it is difficult to measure reagents accurately. (8) K. B. Sharpless and T. R. Verhoeven, Aldrichimica Acta, 12, 63 (1979). This article also contains the best previous result (80% ee) for asymmetric ...
... small excess (2-5%) to be safe. This consideration is more important for small-scale reactions where it is difficult to measure reagents accurately. (8) K. B. Sharpless and T. R. Verhoeven, Aldrichimica Acta, 12, 63 (1979). This article also contains the best previous result (80% ee) for asymmetric ...
Regiospecificity according to Markovnikov
... • Enols rearrange to the isomeric ketone by the rapid transfer of a proton from the hydroxyl to the alkene carbon • The keto form is usually so stable compared to the enol that only the keto form can be observed ...
... • Enols rearrange to the isomeric ketone by the rapid transfer of a proton from the hydroxyl to the alkene carbon • The keto form is usually so stable compared to the enol that only the keto form can be observed ...
The Oxidation States of Tin
... results of the physical observations. These included a melting point test for both compounds and a KI spot test for the tin(IV) iodide. The first test that was done was the characterization test performed with the saturated KI solution. A literature search was performed and it was found that KI will ...
... results of the physical observations. These included a melting point test for both compounds and a KI spot test for the tin(IV) iodide. The first test that was done was the characterization test performed with the saturated KI solution. A literature search was performed and it was found that KI will ...
Chapter 14 - people.vcu.edu
... The nucleophile hits the less substituted side of the epoxide ring. This is because of steric effects. This is true of all bases, not just hydroxide and alkoxides. o There’s no point in treating Grignards and organolithiums differently here because they’re just bases too! You will often see ...
... The nucleophile hits the less substituted side of the epoxide ring. This is because of steric effects. This is true of all bases, not just hydroxide and alkoxides. o There’s no point in treating Grignards and organolithiums differently here because they’re just bases too! You will often see ...
Orbitals - drjosephryan.com
... ketones with hydride reagents may be represented as proceeding through a nucleophilic addition of a hydride ion (:H–) to the C=O carbon • LiAlH4 and NaBH4 act as if they are donors of hydride ion ...
... ketones with hydride reagents may be represented as proceeding through a nucleophilic addition of a hydride ion (:H–) to the C=O carbon • LiAlH4 and NaBH4 act as if they are donors of hydride ion ...
asymmetric alkyne addition to aldehydes
... INTRODUCTION Chiral propargylic alcohols are important compounds, as this structural motif is often found in pharmaceutical compounds as well as natural products and can also serve as versatile synthetic intermediates.1 Although there are many methods available for the preparation of these compounds ...
... INTRODUCTION Chiral propargylic alcohols are important compounds, as this structural motif is often found in pharmaceutical compounds as well as natural products and can also serve as versatile synthetic intermediates.1 Although there are many methods available for the preparation of these compounds ...
NaBH4 Reduction of Vanillin
... Aside from a lack of selectivity, LAH reacts violently with water and other hydroxylic compounds, and reductions using this reagent must be carried out under non‐protic, anhydrous conditions. This not only limits the solvents with which LAH can be used, but it presents greater challenges in th ...
... Aside from a lack of selectivity, LAH reacts violently with water and other hydroxylic compounds, and reductions using this reagent must be carried out under non‐protic, anhydrous conditions. This not only limits the solvents with which LAH can be used, but it presents greater challenges in th ...
Discodermolide

(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.