DEHYDRATION - ALKENE TEST EXERCISES
... DEHYDRATION - ALKENE TEST EXERCISES 1. Give a detailed mechanism for the acid-catalyzed dehydration of cyclohexanol to cyclohexene. ...
... DEHYDRATION - ALKENE TEST EXERCISES 1. Give a detailed mechanism for the acid-catalyzed dehydration of cyclohexanol to cyclohexene. ...
Chapter 20 - Simpson County Schools
... 20.8 PHYSICAL AND CHEMICAL PROPERTIES OF ALKYNES must contain at least one carbon≡carbon (triple bond) gas at room temperature slightly soluble in water but still considered nonpolar Ethyne is commonly called acetylene; used in oxyacetylene cutting/welding torches and as an intermediate in th ...
... 20.8 PHYSICAL AND CHEMICAL PROPERTIES OF ALKYNES must contain at least one carbon≡carbon (triple bond) gas at room temperature slightly soluble in water but still considered nonpolar Ethyne is commonly called acetylene; used in oxyacetylene cutting/welding torches and as an intermediate in th ...
Reaction of Alkenes
... The reaction is regioselective The reaction is regioselective (alcohol on the least‐substituted carbon) (alcohol on the least substituted carbon) and stereoselective (syn‐addition) ...
... The reaction is regioselective The reaction is regioselective (alcohol on the least‐substituted carbon) (alcohol on the least substituted carbon) and stereoselective (syn‐addition) ...
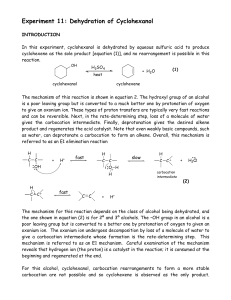
Dehydration of Cyclohexanol
... position of equilibrium will be shifted to the right by continuously removing cyclohexene as it is formed using fractional distillation with a packed fractional distillation column. Fractional distillation also ensures that the cyclohexene product (boiling point = 83°C) is not contaminated with the ...
... position of equilibrium will be shifted to the right by continuously removing cyclohexene as it is formed using fractional distillation with a packed fractional distillation column. Fractional distillation also ensures that the cyclohexene product (boiling point = 83°C) is not contaminated with the ...
Kazzie`s Guide to Orgo 2
... General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 210 Stuff Identify ...
... General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 210 Stuff Identify ...
Glossary of Key Terms in Chapter Two
... addition reaction (11.5) a reaction in which two molecules add together to form a new molecule; often involves the addition of one molecule to a double or triple bond in an unsaturated molecule. alkene (11.1) a hydrocarbon that contains one or more carbon-carbon double bonds; an unsaturated hydrocar ...
... addition reaction (11.5) a reaction in which two molecules add together to form a new molecule; often involves the addition of one molecule to a double or triple bond in an unsaturated molecule. alkene (11.1) a hydrocarbon that contains one or more carbon-carbon double bonds; an unsaturated hydrocar ...
Organic Reactions 2.1- 2.3 - mccormack-sch4u-2013
... •H and X (from H-X) where X= Cl , Br, or I •X and X from (X2) where X= Cl , Br, or I •H and H (from H2) ...
... •H and X (from H-X) where X= Cl , Br, or I •X and X from (X2) where X= Cl , Br, or I •H and H (from H2) ...
Synthesis of a Family of Chiral Asymmetric Schiff - Blogs at H-SC
... trial two. Deprotection yielded spectra that plausibly matched chemical shifts for the intended molecule, even though the product remained an oil. The initial target reaction was not straight out of literature but was rather inspired by work done by Hoveyda’s group. Thus, the target compound, under ...
... trial two. Deprotection yielded spectra that plausibly matched chemical shifts for the intended molecule, even though the product remained an oil. The initial target reaction was not straight out of literature but was rather inspired by work done by Hoveyda’s group. Thus, the target compound, under ...
(substituted) carbon
... Hydroboration-oxidation of alkenes allows stereospecific and regioselective synthesis of alcohols. The reaction sequence exhibits anti-Markovnikov regioselectivity which complements acid-catalyzed hydration and oxymercurationdemercuration. The reaction mechanism does not involve a carbocation and t ...
... Hydroboration-oxidation of alkenes allows stereospecific and regioselective synthesis of alcohols. The reaction sequence exhibits anti-Markovnikov regioselectivity which complements acid-catalyzed hydration and oxymercurationdemercuration. The reaction mechanism does not involve a carbocation and t ...
chapter 2: reactions of organic compounds
... •H and X (from H-X) where X= Cl , Br, or I •X and X from (X2) where X= Cl , Br, or I •H and H (from H2) ...
... •H and X (from H-X) where X= Cl , Br, or I •X and X from (X2) where X= Cl , Br, or I •H and H (from H2) ...
E2 reactions
... Occurs by abstraction of H+ from a C adjacent to the C with the LG. Products follow Zaitsev’s Rule. ...
... Occurs by abstraction of H+ from a C adjacent to the C with the LG. Products follow Zaitsev’s Rule. ...
Nugget
... The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases ...
... The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the inversion have been proposed Our primary goal is to use symmetrically substituted chiral Tröger’s bases ...
document
... The greater number of attached alkyl groups or the more highly substituted the carbon atoms of the double bond, the greater is the alkene’s stability ...
... The greater number of attached alkyl groups or the more highly substituted the carbon atoms of the double bond, the greater is the alkene’s stability ...
Abbreviated Chapter 17 Powerpoint
... • Most electron-withdrawing groups are deactivators and meta-directors. • The atom attached to the aromatic ring has a positive or partial positive charge. • Electron density is withdrawn inductively along the sigma bond, so the ring has less electron density than benzene, and thus it will be slower ...
... • Most electron-withdrawing groups are deactivators and meta-directors. • The atom attached to the aromatic ring has a positive or partial positive charge. • Electron density is withdrawn inductively along the sigma bond, so the ring has less electron density than benzene, and thus it will be slower ...
ADDITION REACTIONS
... He found that, when two products were formed, one was formed in a larger quantity. His original rule was based only on this reaction. The modern version uses carbocation stability as a criterion for predicting the products. In the electrophilic addition to alkenes the major product is formed via the ...
... He found that, when two products were formed, one was formed in a larger quantity. His original rule was based only on this reaction. The modern version uses carbocation stability as a criterion for predicting the products. In the electrophilic addition to alkenes the major product is formed via the ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... b) How will you convert isopropyl alcohol into n- propyl alcohol. ...
... b) How will you convert isopropyl alcohol into n- propyl alcohol. ...
Mechanistic Assignment
... CH3CH2SH is a good Lewis base. Why doesn’t it just react with the Lewis acid (BF3)? You will likely want to refer to your mechanism to explain why that is a better reaction path. ...
... CH3CH2SH is a good Lewis base. Why doesn’t it just react with the Lewis acid (BF3)? You will likely want to refer to your mechanism to explain why that is a better reaction path. ...
Answer Key for Final Exam
... 11. Consider all the data shown below, and determine the structure of the unknown molecule. Assign every peak in the 1H NMR and 13C NMR to atoms in the molecule. Also identify key peak(s) in the IR spectrum and MS spectrum (20 points). Molecular formula: C11H14O2 ...
... 11. Consider all the data shown below, and determine the structure of the unknown molecule. Assign every peak in the 1H NMR and 13C NMR to atoms in the molecule. Also identify key peak(s) in the IR spectrum and MS spectrum (20 points). Molecular formula: C11H14O2 ...
L3 - Alcohol and Phenol Reactions
... oxidized by removing two hydrogen atoms from the same molecule, one from a hydroxyl group and the second from the carbon of the hydroxyl group, resulting in the formation of a double bond between the carbon and remaining oxygen ...
... oxidized by removing two hydrogen atoms from the same molecule, one from a hydroxyl group and the second from the carbon of the hydroxyl group, resulting in the formation of a double bond between the carbon and remaining oxygen ...
Organic Chemistry 1 1st Hour Exam Student ID # Name
... 1. Draw as many skeletal structures of the isomers (constitutional isomers or stereoisomers) for a compound with the molecular formula C5H10 as possible and name each isomer according to the IUPAC nomenclature. You may use the condensed formula to draw the structure if you want. ...
... 1. Draw as many skeletal structures of the isomers (constitutional isomers or stereoisomers) for a compound with the molecular formula C5H10 as possible and name each isomer according to the IUPAC nomenclature. You may use the condensed formula to draw the structure if you want. ...
Assignment 4 Task 1a
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... (i) CH3CHOHCH=CH2 (ii) CH3CH2C(CH3)(OH)CH2CH2CH3 07. COC bond angle in diethyl ether is greater than HOH bond angle in water. Explain. 08. Arrange the following in the order of increasing acidity. p-toluic acid, phenyl acetic acid, m-nitrobenzoic acid, benzoic acid. 09. Accont for the low ...
... (i) CH3CHOHCH=CH2 (ii) CH3CH2C(CH3)(OH)CH2CH2CH3 07. COC bond angle in diethyl ether is greater than HOH bond angle in water. Explain. 08. Arrange the following in the order of increasing acidity. p-toluic acid, phenyl acetic acid, m-nitrobenzoic acid, benzoic acid. 09. Accont for the low ...
Tiffeneau–Demjanov rearrangement

The Tiffeneau–Demjanov rearrangement (TDR) is the chemical reaction of a 1-aminomethyl-cycloalkanol with nitrous acid to form an enlarged cycloketone.The Tiffeneau–Demjanov ring expansion, Tiffeneau–Demjanov rearrangement, or TDR, provides an easy way to increase amino-substituted cycloalkanes and cycloalkanols in size by one carbon. Ring sizes from cyclopropane through cyclooctane are able to undergo Tiffeneau–Demjanov ring expansion with some degree of success. Yields decrease as initial ring size increases, and the ideal use of TDR is for synthesis of five, six, and seven membered rings. A principal synthetic application of Tiffeneau–Demjanov ring expansion is to bicyclic or polycyclic systems. Several reviews on this reaction have been published.