6-organic - fixurscore
... tertiary carbocation is made stabilised by the electron releasing methyl groups around it. (see alkenes topic for another example of this). Also the bulky methyl groups prevent the hydroxide ion from attacking the halogenoalkane in the same way as the mechanism above ...
... tertiary carbocation is made stabilised by the electron releasing methyl groups around it. (see alkenes topic for another example of this). Also the bulky methyl groups prevent the hydroxide ion from attacking the halogenoalkane in the same way as the mechanism above ...
Lecture Resource ()
... Organometallic Compounds An organic compound containing a carbon–metal bond ...
... Organometallic Compounds An organic compound containing a carbon–metal bond ...
Glossary The definitions in this glossary have
... Inert Gas – this can either be nitrogen supplied by an onboard nitrogen plant, or water scrubbed exhaust gas with a high content of nitrogen and carbon dioxide, and with a low content of oxygen. The composition of water scrubbed exhaust gas will depend on the oil used and engine efficiency Inorganic ...
... Inert Gas – this can either be nitrogen supplied by an onboard nitrogen plant, or water scrubbed exhaust gas with a high content of nitrogen and carbon dioxide, and with a low content of oxygen. The composition of water scrubbed exhaust gas will depend on the oil used and engine efficiency Inorganic ...
Chapter 5. An Overview of Organic Reactions
... C-H bonds only (no functional groups) Connecting carbons can lead to large or small molecules The formula for an alkane with no rings in it must be CnH2n+2 where the number of C’s is n Alkanes are saturated with hydrogen (no more can be added They are also called aliphatic compounds ...
... C-H bonds only (no functional groups) Connecting carbons can lead to large or small molecules The formula for an alkane with no rings in it must be CnH2n+2 where the number of C’s is n Alkanes are saturated with hydrogen (no more can be added They are also called aliphatic compounds ...
org test 1
... 2. Why is Sulphuric acid not used during reaction of alcohol with KI? 3. Why is preparation of ethers by acid catalysed dehydration of 2° and 3° alcohols not a suitable method? 4. Of benzene and phenol, which is more easily nitrated and why? 5. Ethers possess a net dipole moment even if they are sym ...
... 2. Why is Sulphuric acid not used during reaction of alcohol with KI? 3. Why is preparation of ethers by acid catalysed dehydration of 2° and 3° alcohols not a suitable method? 4. Of benzene and phenol, which is more easily nitrated and why? 5. Ethers possess a net dipole moment even if they are sym ...
Carboxylic Acid Derivatives
... A similar procedure is used to make amides from acyl chlorides and amines (the amine must have at least one hydrogen attached to the nitrogen). ...
... A similar procedure is used to make amides from acyl chlorides and amines (the amine must have at least one hydrogen attached to the nitrogen). ...
Addition reactions
... Tertiary alcohols are not easily oxidised because, unlike primary and secondary alcohols, they do not have a hydrogen attached to the same carbon atom as the hydroxyl group. Of course, the opposite of oxidation is reduction and the previous two examples can also go in reverse: Example: reduction of ...
... Tertiary alcohols are not easily oxidised because, unlike primary and secondary alcohols, they do not have a hydrogen attached to the same carbon atom as the hydroxyl group. Of course, the opposite of oxidation is reduction and the previous two examples can also go in reverse: Example: reduction of ...
Taylor`s Organic Reactions Summary Sheet
... reactants, and must be considered in designing the synthesis of specific alkyl halides. These alkyl halides can then be transformed into other organic compounds. Preparing Organic Halides: Halogenation ...
... reactants, and must be considered in designing the synthesis of specific alkyl halides. These alkyl halides can then be transformed into other organic compounds. Preparing Organic Halides: Halogenation ...
06 MC /08 MC /08 NMR
... Counting this cover sheet, there are a total of 9 pages- check to ensure you have all 9. All 9 pages must be turned in for grading. Use the backside of preceding pages for scratch paper. Any cheating will result in the dismissal from c/ass with an "F" grade. Please put your name or initial on each p ...
... Counting this cover sheet, there are a total of 9 pages- check to ensure you have all 9. All 9 pages must be turned in for grading. Use the backside of preceding pages for scratch paper. Any cheating will result in the dismissal from c/ass with an "F" grade. Please put your name or initial on each p ...
... around the ring. Yields are excellent (>75%) in almost all cases, except for R3 = CO2Et (50%) and R3 = H (42%). The stability of halogen substituents (R1 = Cl, Br) to the reaction conditions, providing a handle for further functionalization, is worthy of note. Two examples of spirocyclic dihydrobenz ...
Carboxylic Acid Derivatives
... If it is A AC2 (with Nu), the intermediate and reactant (the protonated starting material) are both positively charged. Electronegative groups would destabilize both. However, the effect may be greater on the reactant because the positive charge is transferred to the carbonyl carbon by both an induc ...
... If it is A AC2 (with Nu), the intermediate and reactant (the protonated starting material) are both positively charged. Electronegative groups would destabilize both. However, the effect may be greater on the reactant because the positive charge is transferred to the carbonyl carbon by both an induc ...
ALKENES and SULPHURIC ACID
... one of the carbon atoms, and the rest joins on to the other one. Make sure that you can see how the structure of the sulphuric acid relates to the various ways of writing the formula for the product. Important! Learn this structure for sulphuric acid. Sketch it over and over again until you can't po ...
... one of the carbon atoms, and the rest joins on to the other one. Make sure that you can see how the structure of the sulphuric acid relates to the various ways of writing the formula for the product. Important! Learn this structure for sulphuric acid. Sketch it over and over again until you can't po ...
Organic Chemistry
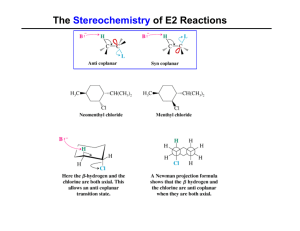
... Q4. Explain why, CH3CH2I loses of HI faster than CD3CH2I loses DI in the presence of a strong base? Ans4. The reaction is an E2 elimination reaction. The (C - H) or (C - D) bond is being broken in the rate determining step. Since the C - H bond is broken at a faster rate than the C - D bond. Therefo ...
... Q4. Explain why, CH3CH2I loses of HI faster than CD3CH2I loses DI in the presence of a strong base? Ans4. The reaction is an E2 elimination reaction. The (C - H) or (C - D) bond is being broken in the rate determining step. Since the C - H bond is broken at a faster rate than the C - D bond. Therefo ...
Islamic University of Gaza
... Indicate if the following statements are true ( √ ) or false ( X) ...
... Indicate if the following statements are true ( √ ) or false ( X) ...
10. Alcohols - The Student Room
... When writing the formulae of aldehydes in a condensed way wire CHO and not COH e.g.CH3CH2CHO Full Oxidation of Primary Alcohols ...
... When writing the formulae of aldehydes in a condensed way wire CHO and not COH e.g.CH3CH2CHO Full Oxidation of Primary Alcohols ...
organic lab questions
... Write descriptive organic equations for all reactions. If no reaction occurred, write N.R. for the products of the reaction. Under each alcohol, write the common name of the alcohol. Under each carboxylic acid write the I.U.P.A.C. name of the carboxylic acid. Finally, write the name of the ester und ...
... Write descriptive organic equations for all reactions. If no reaction occurred, write N.R. for the products of the reaction. Under each alcohol, write the common name of the alcohol. Under each carboxylic acid write the I.U.P.A.C. name of the carboxylic acid. Finally, write the name of the ester und ...
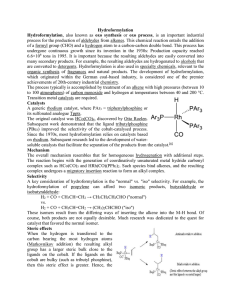
Hydroformylation Hydroformylation, also known as oxo synthesis or
... process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in the 1930s: Production capacity reached 6.6×106 tons in 199 ...
... process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in the 1930s: Production capacity reached 6.6×106 tons in 199 ...
1 - contentextra
... Secondary carbon atom A carbon atom that is attached to the functional group and also to one hydrogen atom. SN1 Substitution nucleophilic unimolecular. A substitution reaction in which a nucleophile attacks a molecule and the mechanism involves a single reactant in the rate-determining step. It is c ...
... Secondary carbon atom A carbon atom that is attached to the functional group and also to one hydrogen atom. SN1 Substitution nucleophilic unimolecular. A substitution reaction in which a nucleophile attacks a molecule and the mechanism involves a single reactant in the rate-determining step. It is c ...
Microsoft Word - Final Exam Study Guide
... equilibria, pKa’s, trends in acidity/basicity, functional groups, alkane nomenclature, conformational analysis, Newman projections, causes of strain, cyclohexane ring ...
... equilibria, pKa’s, trends in acidity/basicity, functional groups, alkane nomenclature, conformational analysis, Newman projections, causes of strain, cyclohexane ring ...
Organic Chemistry (HL) Revision Questions
... Two compounds, A and D, each have the formula C4H9Cl. Compound A is reacted with dilute aqueous sodium hydroxide to produce compound B with a formula of C4H10O. Compound B is then oxidized with acidified potassium manganate(VII) to produce compound C with a formula of C4H8O. Compound C resists furth ...
... Two compounds, A and D, each have the formula C4H9Cl. Compound A is reacted with dilute aqueous sodium hydroxide to produce compound B with a formula of C4H10O. Compound B is then oxidized with acidified potassium manganate(VII) to produce compound C with a formula of C4H8O. Compound C resists furth ...
2015 CH 420 Take Home Quiz 3 March 24
... an arrow to the more nucleophilic nitrogen atom in the hydrazine substrate. In addition, circle the most electrophilic carbonyl in the 1,3-diketone substrate. ...
... an arrow to the more nucleophilic nitrogen atom in the hydrazine substrate. In addition, circle the most electrophilic carbonyl in the 1,3-diketone substrate. ...
Tiffeneau–Demjanov rearrangement

The Tiffeneau–Demjanov rearrangement (TDR) is the chemical reaction of a 1-aminomethyl-cycloalkanol with nitrous acid to form an enlarged cycloketone.The Tiffeneau–Demjanov ring expansion, Tiffeneau–Demjanov rearrangement, or TDR, provides an easy way to increase amino-substituted cycloalkanes and cycloalkanols in size by one carbon. Ring sizes from cyclopropane through cyclooctane are able to undergo Tiffeneau–Demjanov ring expansion with some degree of success. Yields decrease as initial ring size increases, and the ideal use of TDR is for synthesis of five, six, and seven membered rings. A principal synthetic application of Tiffeneau–Demjanov ring expansion is to bicyclic or polycyclic systems. Several reviews on this reaction have been published.