Name__________________________Review Organic Reactions
... 7. When methane reacts with a halogen, the type of reaction is A) addition C) substitution ...
... 7. When methane reacts with a halogen, the type of reaction is A) addition C) substitution ...
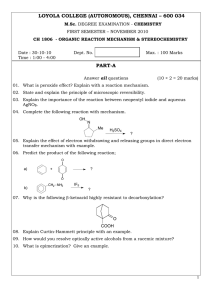
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... CH 1806 - ORGANIC REACTION MECHANISM & STEREOCHEMISTRY ...
... CH 1806 - ORGANIC REACTION MECHANISM & STEREOCHEMISTRY ...
Document
... Give the structural formula of the monomer (containing two functional groups) that could be used to make this polyester and state the names of the two functional groups. ...
... Give the structural formula of the monomer (containing two functional groups) that could be used to make this polyester and state the names of the two functional groups. ...
chemistry pretest - the Biology Scholars Program Wiki
... taken these courses, I do not expect you to be up to speed with all of these questions. However, it is extremely important that you become comfortable with them as soon as possible and I would like to help you with that before the new content kicks in full speed. Therefore, I would like you to work ...
... taken these courses, I do not expect you to be up to speed with all of these questions. However, it is extremely important that you become comfortable with them as soon as possible and I would like to help you with that before the new content kicks in full speed. Therefore, I would like you to work ...
C h e m g u i d e ... ALCOHOLS: THE REACTION WITH SODIUM
... a) Why is it important to test the pH of the liquid before adding the sodium? b) What would you observe if the liquid was actually an alcohol? c) Observing this isn’t enough to be sure that you have an alcohol. What else might cause the result you described in part (b)? d) Suppose you added a small ...
... a) Why is it important to test the pH of the liquid before adding the sodium? b) What would you observe if the liquid was actually an alcohol? c) Observing this isn’t enough to be sure that you have an alcohol. What else might cause the result you described in part (b)? d) Suppose you added a small ...
Document
... A very important addition/elimination reaction • Aspirin is made by an addition/elimination reaction. • The phenol attack the acid anhydride (slightly less reactive than acid chloride but still very reactive). • As usual a tetrahedral intermediate is generated. • This intermediate can regenerate th ...
... A very important addition/elimination reaction • Aspirin is made by an addition/elimination reaction. • The phenol attack the acid anhydride (slightly less reactive than acid chloride but still very reactive). • As usual a tetrahedral intermediate is generated. • This intermediate can regenerate th ...
Reactions of Hydrocarbons & their functional groups
... water adds to a bond splitting it into two Reverse of a condensation reaction Water can add to an ester or amide bond Ester + water makes a carboxylic acid and alcohol Amide + water makes a carboxylic acid and amine ...
... water adds to a bond splitting it into two Reverse of a condensation reaction Water can add to an ester or amide bond Ester + water makes a carboxylic acid and alcohol Amide + water makes a carboxylic acid and amine ...
Notes
... Substitution Reactions ‐ A substitution reaction occurs when a saturated hydrocarbon (alkane) or aromatic reacts with a diatomic halide molecule (like Br2, Cl2, F2, I2) ‐ The products of a substitution reaction are a organic halide and a hydrogen halide molecule ‐ Carbon‐hydrogen bonds in the hydr ...
... Substitution Reactions ‐ A substitution reaction occurs when a saturated hydrocarbon (alkane) or aromatic reacts with a diatomic halide molecule (like Br2, Cl2, F2, I2) ‐ The products of a substitution reaction are a organic halide and a hydrogen halide molecule ‐ Carbon‐hydrogen bonds in the hydr ...
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015
... Major product formed via more stabilized carbocation (Markovnikoff) ...
... Major product formed via more stabilized carbocation (Markovnikoff) ...
Homework #7, Graded Answers
... No reaction tertiary alcohols do not oxidize 31.) Each of the following conversions requires more than one step. Show the reagents you would use and draw structural formulas for intermediate compounds formed in each ...
... No reaction tertiary alcohols do not oxidize 31.) Each of the following conversions requires more than one step. Show the reagents you would use and draw structural formulas for intermediate compounds formed in each ...
Worksheet 10.1
... Amine A homologous series containing the functional group –NH2. Secondary and tertiary amines respectively have one or two of the hydrogen atoms substituted by alkyl groups. Carbocation A species formed as an intermediate during a reaction that has a positive charge on a carbon atom. Tertiary carboc ...
... Amine A homologous series containing the functional group –NH2. Secondary and tertiary amines respectively have one or two of the hydrogen atoms substituted by alkyl groups. Carbocation A species formed as an intermediate during a reaction that has a positive charge on a carbon atom. Tertiary carboc ...
Eliminations
... So far we have discussed only one reaction type: substitutions. We now introduce a new reaction type: eliminations. They are related to substitution reactions and often accompany them. Instead of substituting ...
... So far we have discussed only one reaction type: substitutions. We now introduce a new reaction type: eliminations. They are related to substitution reactions and often accompany them. Instead of substituting ...
Final Exam Review Sheet Chemistry 110a/1998
... cation, and anion using a resonance and molecular orbital argument. How does the allylic radical compare in stability to 3°, 2°, and 1°? How about the allylic cation, in this regard? The pKa of an allylic hydrogen is 41: how can you use this value to say that the allylic anion is more stable than th ...
... cation, and anion using a resonance and molecular orbital argument. How does the allylic radical compare in stability to 3°, 2°, and 1°? How about the allylic cation, in this regard? The pKa of an allylic hydrogen is 41: how can you use this value to say that the allylic anion is more stable than th ...
3.8 ADDITION OF WATER TO AN ALKENE H or enzyme + H-O
... Our example just shows one molecule of monomer as a reactant, although in fact there are large numbers of them that will react with each other to form the polymer: The one sided arrows indicate that one electron goes to each C atom. This is in contrast to the previous reaction pathways where both el ...
... Our example just shows one molecule of monomer as a reactant, although in fact there are large numbers of them that will react with each other to form the polymer: The one sided arrows indicate that one electron goes to each C atom. This is in contrast to the previous reaction pathways where both el ...
2010-09-16 Alcohols
... The simple way to think of alcohols is that there is a water molecule where the hydrogen at one of the termini is replaced by a carbon chain. The alcohol present in beverages is ethanol, which is the mildest depressant and narcotic poison of all alcohols. Other alcohols magnify this effect and are ...
... The simple way to think of alcohols is that there is a water molecule where the hydrogen at one of the termini is replaced by a carbon chain. The alcohol present in beverages is ethanol, which is the mildest depressant and narcotic poison of all alcohols. Other alcohols magnify this effect and are ...
Chapter 7
... atoms in the T.S. of an E2 reaction must lie in the same plane. • There are two ways this can happen: ...
... atoms in the T.S. of an E2 reaction must lie in the same plane. • There are two ways this can happen: ...
REACTIONS OF ALCOHOLS
... • This reaction with the Lucas Reagent (ZnCl2) is a qualitative test for the different types of alcohols because the rate of the reaction differs greatly for a primary, secondary and tertiary alcohol. • The difference in rates is due to the solubility of the resulting alkyl halides • Tertiary Alcoho ...
... • This reaction with the Lucas Reagent (ZnCl2) is a qualitative test for the different types of alcohols because the rate of the reaction differs greatly for a primary, secondary and tertiary alcohol. • The difference in rates is due to the solubility of the resulting alkyl halides • Tertiary Alcoho ...
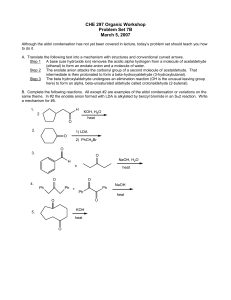
CHE 297 Organic Workshop
... Problem Set 7B March 5, 2007 Although the aldol condensation has not yet been covered in lecture, today’s problem set should teach you how to do it. A. Translate the following text into a mechanism with structures and conventional curved arrows. Step 1 A base (use hydroxide ion) removes the acidic a ...
... Problem Set 7B March 5, 2007 Although the aldol condensation has not yet been covered in lecture, today’s problem set should teach you how to do it. A. Translate the following text into a mechanism with structures and conventional curved arrows. Step 1 A base (use hydroxide ion) removes the acidic a ...
Exam 2 SOLUTION
... and used for scratch paper. Bring all materials up when you are finished with the exam. Good luck! 1. Predict the product, or give the starting material for the following reactions: [12] ...
... and used for scratch paper. Bring all materials up when you are finished with the exam. Good luck! 1. Predict the product, or give the starting material for the following reactions: [12] ...
Chem 30B Spring 2004 QUIZ #1 KEY Weds April 14th / 30
... step of the mechanism involves the formation of a species called a chromate ester – what is the oxidation state of the Cr atom in this species? A ...
... step of the mechanism involves the formation of a species called a chromate ester – what is the oxidation state of the Cr atom in this species? A ...
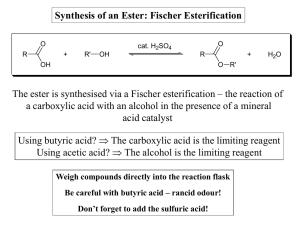
Synthesis of an Ester: Fischer Esterification The ester is synthesised
... As a result, they are partially extracted into the aqueous layer during work-up, reducing the amount recovered. ...
... As a result, they are partially extracted into the aqueous layer during work-up, reducing the amount recovered. ...
Practice Questions for Chapters 1-8 CHEM 4000A
... In the first step, a relatively acidic site (pKa ~10) is deprotonated. The negative charge is delocalized onto two different oxygen atoms, stabilizing the anion shown. In the second step, the nucleophilic carbanion attacks the electrophilic carbon of the thioester. A tetrahedral intermediate is form ...
... In the first step, a relatively acidic site (pKa ~10) is deprotonated. The negative charge is delocalized onto two different oxygen atoms, stabilizing the anion shown. In the second step, the nucleophilic carbanion attacks the electrophilic carbon of the thioester. A tetrahedral intermediate is form ...
Preface - Wiley Online Library
... atom. Nitrogen boasts the strongest homoatomic bond rendering it chemically rather inert, but yet its fixation into plant material can be accomplished under the mildest of conditions. In his marvelous book, The Disappearing Spoon, Sean McKean eloquently summed up the idiosyncratic character of nitro ...
... atom. Nitrogen boasts the strongest homoatomic bond rendering it chemically rather inert, but yet its fixation into plant material can be accomplished under the mildest of conditions. In his marvelous book, The Disappearing Spoon, Sean McKean eloquently summed up the idiosyncratic character of nitro ...
Tiffeneau–Demjanov rearrangement

The Tiffeneau–Demjanov rearrangement (TDR) is the chemical reaction of a 1-aminomethyl-cycloalkanol with nitrous acid to form an enlarged cycloketone.The Tiffeneau–Demjanov ring expansion, Tiffeneau–Demjanov rearrangement, or TDR, provides an easy way to increase amino-substituted cycloalkanes and cycloalkanols in size by one carbon. Ring sizes from cyclopropane through cyclooctane are able to undergo Tiffeneau–Demjanov ring expansion with some degree of success. Yields decrease as initial ring size increases, and the ideal use of TDR is for synthesis of five, six, and seven membered rings. A principal synthetic application of Tiffeneau–Demjanov ring expansion is to bicyclic or polycyclic systems. Several reviews on this reaction have been published.