Jeopardy
... What is the name of the rule that states that the nucleophilic part of the reagent will bind with the most stable carbon of the substrate? ...
... What is the name of the rule that states that the nucleophilic part of the reagent will bind with the most stable carbon of the substrate? ...
Guideline
... a tertiary carbocation over a secondary, which in turn is more stable than a primary. Generally, the largest substituent at a branch is eliminated most readily as a radical, presumably because a long-chain radical can achieve some stability by delocalization of the lone electron. 4. Double bonds, cy ...
... a tertiary carbocation over a secondary, which in turn is more stable than a primary. Generally, the largest substituent at a branch is eliminated most readily as a radical, presumably because a long-chain radical can achieve some stability by delocalization of the lone electron. 4. Double bonds, cy ...
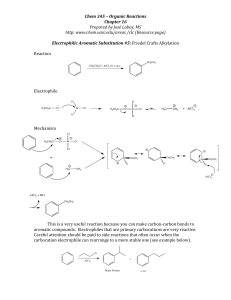
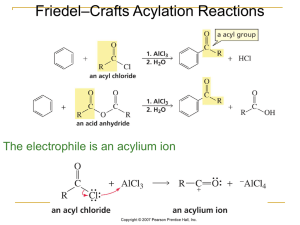
EAS Friedel-Crafts Alkylation
... aromatic compounds. Electrophiles that are primary carbocations are very reactive. Careful attention should be paid to side reactions that often occur when the carbocation electrophile can rearrange to a ...
... aromatic compounds. Electrophiles that are primary carbocations are very reactive. Careful attention should be paid to side reactions that often occur when the carbocation electrophile can rearrange to a ...
chapter 8 part 2
... carbon atom of the double bond The more substituted carbon bears an electron-releasing alkyl group, it is better able to accommodate this positive charge ...
... carbon atom of the double bond The more substituted carbon bears an electron-releasing alkyl group, it is better able to accommodate this positive charge ...
Slide 1
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation rearrangement ...
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation rearrangement ...
Alkenes
... The 2s electron and two of the 2p electrons combine to form three sp2 hybrid orbitals, leaving a spare porbital on each of the carbon atoms ...
... The 2s electron and two of the 2p electrons combine to form three sp2 hybrid orbitals, leaving a spare porbital on each of the carbon atoms ...
Answer on Question#52196 - Chemistry
... The anti-Markovnikov rule can be best explained by taking an example of addition of hydrogen bromide to propene in the presence of benzoyl peroxide. The reaction of HBr with substituted alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the ...
... The anti-Markovnikov rule can be best explained by taking an example of addition of hydrogen bromide to propene in the presence of benzoyl peroxide. The reaction of HBr with substituted alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the ...
Fundamentals Of Organic Chemistry
... a 1° carbocation. The 1° carbocation undergo rearrangement to form more stable 3° carbocation by 1, 2-alkyl shift before the product is formed. Finally occurs and products are formed according to saytzeff's rule. ...
... a 1° carbocation. The 1° carbocation undergo rearrangement to form more stable 3° carbocation by 1, 2-alkyl shift before the product is formed. Finally occurs and products are formed according to saytzeff's rule. ...
Assignment 2 Group A and B
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
Chapter Nine: Alcohols, Ethers and Epoxides
... iii. Reduction with hydride reducing agent LiAlH4 : 12.6 ...
... iii. Reduction with hydride reducing agent LiAlH4 : 12.6 ...
AMINO ACIDS Ethan Secor, John N. Gitua (Mentor)
... Ethan Secor, John N. Gitua (Mentor) Department of Chemistry, College of Arts and Sciences Drake University RESULTS ...
... Ethan Secor, John N. Gitua (Mentor) Department of Chemistry, College of Arts and Sciences Drake University RESULTS ...
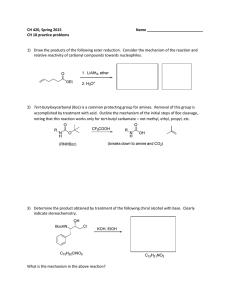
CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
... 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ...
... 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ...
$doc.title
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
Tiffeneau–Demjanov rearrangement

The Tiffeneau–Demjanov rearrangement (TDR) is the chemical reaction of a 1-aminomethyl-cycloalkanol with nitrous acid to form an enlarged cycloketone.The Tiffeneau–Demjanov ring expansion, Tiffeneau–Demjanov rearrangement, or TDR, provides an easy way to increase amino-substituted cycloalkanes and cycloalkanols in size by one carbon. Ring sizes from cyclopropane through cyclooctane are able to undergo Tiffeneau–Demjanov ring expansion with some degree of success. Yields decrease as initial ring size increases, and the ideal use of TDR is for synthesis of five, six, and seven membered rings. A principal synthetic application of Tiffeneau–Demjanov ring expansion is to bicyclic or polycyclic systems. Several reviews on this reaction have been published.