Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
... produced artificially, and also occurring naturally in beet-root molasses and its residues. The listed pronunciation indicates it has the exact same emphasis as “cocaine”. Cocaine \Co"ca*ine\, n. (Chem.) A powerful alkaloid, {C17H21NO4}, obtained from the leaves of coca So – if you say “co-ca-ee ...
... produced artificially, and also occurring naturally in beet-root molasses and its residues. The listed pronunciation indicates it has the exact same emphasis as “cocaine”. Cocaine \Co"ca*ine\, n. (Chem.) A powerful alkaloid, {C17H21NO4}, obtained from the leaves of coca So – if you say “co-ca-ee ...
Alkenes 3 - ChemWeb (UCC)
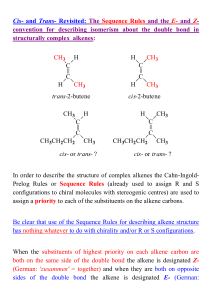
... This reaction illustrated above is called a 1,2- or -elimination to indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the act ...
... This reaction illustrated above is called a 1,2- or -elimination to indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the act ...
carbonyl compound group
... −I effect. Thus, acids containing Br− are stronger. Now, the +I effect of isopropyl group is more than that of n-propyl group. Hence, (CH3)2CHCOOH is a weaker acid than CH3CH2CH2COOH. Also, the −I effect grows weaker as distance increases. Hence, CH3CH(Br)CH2COOH is a ...
... −I effect. Thus, acids containing Br− are stronger. Now, the +I effect of isopropyl group is more than that of n-propyl group. Hence, (CH3)2CHCOOH is a weaker acid than CH3CH2CH2COOH. Also, the −I effect grows weaker as distance increases. Hence, CH3CH(Br)CH2COOH is a ...
Chapter 21 Carboxylic Acid Derivatives
... Naming Amides • For 1 amide, drop -ic or -oic acid from the carboxylic acid name, add -amide. • For 2 and 3 amides, the alkyl groups bonded to nitrogen are named with Nto indicate their position. O CH3 CH3CHC N ...
... Naming Amides • For 1 amide, drop -ic or -oic acid from the carboxylic acid name, add -amide. • For 2 and 3 amides, the alkyl groups bonded to nitrogen are named with Nto indicate their position. O CH3 CH3CHC N ...
PDF document
... of chemicals having a certain oxidising power. Reactions of benzylic alcohols with F-TEDA-BF4 were studied and their oxidation to benzaldehyde derivatives established.24 Reactions in acetonitrile were found, at least for derivatives bearing a deactivated aromatic ring, to be relatively slow (Table 2 ...
... of chemicals having a certain oxidising power. Reactions of benzylic alcohols with F-TEDA-BF4 were studied and their oxidation to benzaldehyde derivatives established.24 Reactions in acetonitrile were found, at least for derivatives bearing a deactivated aromatic ring, to be relatively slow (Table 2 ...
Get PDF - Wiley Online Library
... rearranged products arising from preferential reaction at the less hindered alkene p-face.[12] This material was subjected directly to reduction with LiAlH4 to deliver alcohol 12 in 63 % yield from 11. Finally, hydrolysis of the TBS ether and double oxidation under Swern conditions gave the targeted ...
... rearranged products arising from preferential reaction at the less hindered alkene p-face.[12] This material was subjected directly to reduction with LiAlH4 to deliver alcohol 12 in 63 % yield from 11. Finally, hydrolysis of the TBS ether and double oxidation under Swern conditions gave the targeted ...
Document
... 37) Tertiary alcohols cannot be oxidized because A) there are no oxygen atoms to remove from the alcohol carbon. B) there are no hydrogen atoms attached to the alcohol carbon. C) the alcohol carbon is bonded to four groups so no oxygen can be added to it. D) the alcohol carbon is bonded to four gro ...
... 37) Tertiary alcohols cannot be oxidized because A) there are no oxygen atoms to remove from the alcohol carbon. B) there are no hydrogen atoms attached to the alcohol carbon. C) the alcohol carbon is bonded to four groups so no oxygen can be added to it. D) the alcohol carbon is bonded to four gro ...
as a PDF
... Currently Indonesia is the world largest palm oil producer with production volume reaching 16 million tones per annum. The high crude oil and ethylene prices in the last 3 – 4 years contribute to the healthy demand growth for basic oleochemicals: fatty acids and fatty alcohols. Oleochemicals are sta ...
... Currently Indonesia is the world largest palm oil producer with production volume reaching 16 million tones per annum. The high crude oil and ethylene prices in the last 3 – 4 years contribute to the healthy demand growth for basic oleochemicals: fatty acids and fatty alcohols. Oleochemicals are sta ...
Chapter 20: Carboxylic Acids and Nitriles
... Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
... Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
98 pts
... (8 pts) Mark as true (T) or false (F) the following statements. Do not explain! • (T) All E1 reactions involve formation of carbocations; • (T) More stable carbocations are generated faster; • (T) Carbocations are electrophiles; • (T) Carbocations are electron deficient; • (T) Free radicals are elec ...
... (8 pts) Mark as true (T) or false (F) the following statements. Do not explain! • (T) All E1 reactions involve formation of carbocations; • (T) More stable carbocations are generated faster; • (T) Carbocations are electrophiles; • (T) Carbocations are electron deficient; • (T) Free radicals are elec ...
Chapter 8 I. Nucleophilic Substitution
... rate = k [CH3Br] [HO – ] What is the reaction order of each starting material? What can you infer on a molecular level? What is the overall order of reaction? ...
... rate = k [CH3Br] [HO – ] What is the reaction order of each starting material? What can you infer on a molecular level? What is the overall order of reaction? ...
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
... Be able to draw by using a perspective formula, Newman projections and Fischer projections. ...
... Be able to draw by using a perspective formula, Newman projections and Fischer projections. ...
amines
... of the corresponding alkane or arene. The final -e of the parent hydrocarbon is retained. ...
... of the corresponding alkane or arene. The final -e of the parent hydrocarbon is retained. ...
PDF aldehydes and ketones
... conversion of a planar sp2 centre to a tetrahedral sp3 with an increase in the steric bulk of the intermediate. The preferred direction of approach of the nucleophile to the carbonyl carbon is along an axis through C and O atoms and at an angle of 108o to the plane of the carbonyl group. Addition of ...
... conversion of a planar sp2 centre to a tetrahedral sp3 with an increase in the steric bulk of the intermediate. The preferred direction of approach of the nucleophile to the carbonyl carbon is along an axis through C and O atoms and at an angle of 108o to the plane of the carbonyl group. Addition of ...
CHAPTER V Fischer-Tropsch Syncrude
... the carbon number distribution from C3 to C12, while the second α-value (α2) describes the distribution of the C20 and heavier fraction.(4) This seems to be a mathematical convenience, since the α-value seems to slowly increase with chain length. Some attribute this to short chain olefin incorporati ...
... the carbon number distribution from C3 to C12, while the second α-value (α2) describes the distribution of the C20 and heavier fraction.(4) This seems to be a mathematical convenience, since the α-value seems to slowly increase with chain length. Some attribute this to short chain olefin incorporati ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.