Guidelines on the Use of Disinfectants
... 5.2 Avoid touching the eyes. If bleach gets into the eyes, immediately rinse with water for at least 15 minutes, and consult a physician. 5.3 Bleach should not be used together with, or mixed with, other household detergents because this reduces its effectiveness and can cause chemical reactions. 5. ...
... 5.2 Avoid touching the eyes. If bleach gets into the eyes, immediately rinse with water for at least 15 minutes, and consult a physician. 5.3 Bleach should not be used together with, or mixed with, other household detergents because this reduces its effectiveness and can cause chemical reactions. 5. ...
Amines and amides
... found e.g putrescine [H2N(CH2)4NH2] and cadaverine [H2N(CH2)5NH2]! These are the amines responsible for the terrible smell of rotting meat. 2. Key properties: If it smells of “fish” or “rotting flesh” chances are you have an amine!! Amines behave in a similar way to NH3 but their behaviour is modifi ...
... found e.g putrescine [H2N(CH2)4NH2] and cadaverine [H2N(CH2)5NH2]! These are the amines responsible for the terrible smell of rotting meat. 2. Key properties: If it smells of “fish” or “rotting flesh” chances are you have an amine!! Amines behave in a similar way to NH3 but their behaviour is modifi ...
Chapter Seven PPT
... Chapter 7: Alkenes and Alkynes • Hydrocarbons Containing Double and Triple Bonds • Unsaturated Compounds (Less than Maximum H Atoms) • Alkenes also Referred to as Olefins • Properties Similar to those of Corresponding Alkanes ...
... Chapter 7: Alkenes and Alkynes • Hydrocarbons Containing Double and Triple Bonds • Unsaturated Compounds (Less than Maximum H Atoms) • Alkenes also Referred to as Olefins • Properties Similar to those of Corresponding Alkanes ...
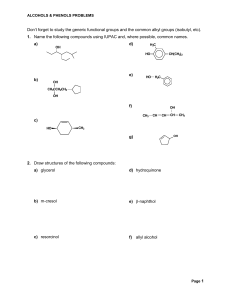
Don`t forget to study the generic functional groups and the common
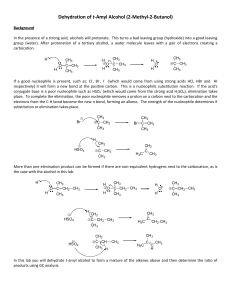
... 11. Write equations to show how the following transformation can be carried out. More than one step may be necessary. There are marks assigned for each intermediate product (not charged transition states) and there are marks for the reagents used; so list them all. No marks are assigned for mechanis ...
... 11. Write equations to show how the following transformation can be carried out. More than one step may be necessary. There are marks assigned for each intermediate product (not charged transition states) and there are marks for the reagents used; so list them all. No marks are assigned for mechanis ...
Carbohydrates: Occurrence, Structures and Chemistry
... replacement of one or more hydroxyl group (s) by a hydrogen atom, an amino group, a thiol group, or similar heteroatomic groups. A similarly broad meaning applies to the word ‘sugar’, which is often used as a synonym for ‘monosaccharide’, but may also be applied to simple compounds containing more t ...
... replacement of one or more hydroxyl group (s) by a hydrogen atom, an amino group, a thiol group, or similar heteroatomic groups. A similarly broad meaning applies to the word ‘sugar’, which is often used as a synonym for ‘monosaccharide’, but may also be applied to simple compounds containing more t ...
carbonyl compounds
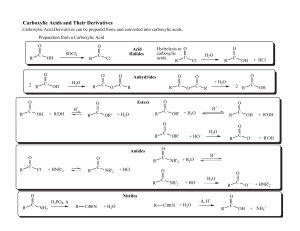
... They react with carbonates and the fizzing produced can be used to distinguish carboxylic acids from other organic compounds 2CH3COOH + CaCO3 (CH3COO)2Ca + H2O + CO2 Ethanoic acid calcium ethanoate ...
... They react with carbonates and the fizzing produced can be used to distinguish carboxylic acids from other organic compounds 2CH3COOH + CaCO3 (CH3COO)2Ca + H2O + CO2 Ethanoic acid calcium ethanoate ...
DEVELOPMENT OF CORRELATIONS FOR DESCRIBING SOLUTE
... Ostwald partition coefficient into hexadecane at 298 K. The regression coefficients and constants (c, e, s, a, b, v and l) are obtained by regression analysis of experimental data for a specific process (i.e., a given partitioning process, for GLC or HPLC processes, a given stationary phase and mobi ...
... Ostwald partition coefficient into hexadecane at 298 K. The regression coefficients and constants (c, e, s, a, b, v and l) are obtained by regression analysis of experimental data for a specific process (i.e., a given partitioning process, for GLC or HPLC processes, a given stationary phase and mobi ...
Alkenes
... The Zaitsev Rule Zaitsev Rule states that the elimination reaction yields the more highly substituted alkene as the major product. The more stable alkene product predominates. ...
... The Zaitsev Rule Zaitsev Rule states that the elimination reaction yields the more highly substituted alkene as the major product. The more stable alkene product predominates. ...
Amines By
... Primary: the “R” is only bonded in one place in this type of bond Secondary: The “R” is bonded in two places in this bond Tertiary: The “R” is bonded in three places in this bond. ...
... Primary: the “R” is only bonded in one place in this type of bond Secondary: The “R” is bonded in two places in this bond Tertiary: The “R” is bonded in three places in this bond. ...
organic families
... Use these results to identify the functional group - alcohol, carboxylic acid or ester. P ...
... Use these results to identify the functional group - alcohol, carboxylic acid or ester. P ...
MS PowerPoint - Catalysis Eprints database
... LLDPE- IFP & SABIC processes Dimerization of propylene- Methyl pentenes & HexenesGasoline additives- DIMERSOL- IFP Sumitomo & BP ...
... LLDPE- IFP & SABIC processes Dimerization of propylene- Methyl pentenes & HexenesGasoline additives- DIMERSOL- IFP Sumitomo & BP ...
View/Open
... © 2008 Thomson Learning, Inc. All Rights Reserved. Thomson Learning WebTutorTM is a trademark of Thomson Learning, Inc. Library of Congress Control Number: 2006938700 ...
... © 2008 Thomson Learning, Inc. All Rights Reserved. Thomson Learning WebTutorTM is a trademark of Thomson Learning, Inc. Library of Congress Control Number: 2006938700 ...
Reprinted from The Journal of Physical Chemistry, lgg5, 99
... STM images of n-alcohols adsorbedon graphite indicate that the OH functional group cannot be distinguished from the methylene groups of the hydrocarbon chain attachedto the OH group.lb'2'3On the other hand, much like the aromatic rings in liquid crystalsdescribedabove,5'hsulfur atoms in the long cha ...
... STM images of n-alcohols adsorbedon graphite indicate that the OH functional group cannot be distinguished from the methylene groups of the hydrocarbon chain attachedto the OH group.lb'2'3On the other hand, much like the aromatic rings in liquid crystalsdescribedabove,5'hsulfur atoms in the long cha ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.