Single Replacement Reactions
... with excess water and inform the instructor. Wear safety goggles and closed toed shoes throughout the entirety of the lab procedure.*** b. Label five test tubes - each with the name of one of the metals (zinc, aluminum, copper, iron and magnesium) if this has not been done already. c. Following your ...
... with excess water and inform the instructor. Wear safety goggles and closed toed shoes throughout the entirety of the lab procedure.*** b. Label five test tubes - each with the name of one of the metals (zinc, aluminum, copper, iron and magnesium) if this has not been done already. c. Following your ...
Oxoacids of Phosphorus
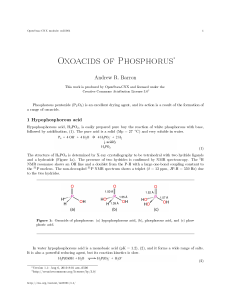
... The reaction of P4 O6 or PCl3 with water yields phosphorous acid, H3 PO3 ; which like hypophosphorous acid is a solid (Mp = 70.1 ◦ C) and very soluble in water. The structure is shown by X-ray crystallography to be comprised of a tetrahedral phosphorus with one hydride and two hydroxides (Figure 1b) ...
... The reaction of P4 O6 or PCl3 with water yields phosphorous acid, H3 PO3 ; which like hypophosphorous acid is a solid (Mp = 70.1 ◦ C) and very soluble in water. The structure is shown by X-ray crystallography to be comprised of a tetrahedral phosphorus with one hydride and two hydroxides (Figure 1b) ...
F324 : Rings, Polymers and Analysis
... explain that NMR spectroscopy is the same technology as that used in ‘magnetic resonance imaging’ (MRI) to obtain diagnostic information about internal structures in body scanners; (i) For organic compounds containing any of the following atoms: C, H, N and O: (i) analyse infrared absorptions in an ...
... explain that NMR spectroscopy is the same technology as that used in ‘magnetic resonance imaging’ (MRI) to obtain diagnostic information about internal structures in body scanners; (i) For organic compounds containing any of the following atoms: C, H, N and O: (i) analyse infrared absorptions in an ...
Organic Chemistry
... • Hydrocarbons that contain one triple bond between carbon atoms • Called “unsaturated hydrocarbon” because it does not have the maximum number of hydrogen atoms • All alkynes end in - yne • Prefixes are the same as alkanes and ...
... • Hydrocarbons that contain one triple bond between carbon atoms • Called “unsaturated hydrocarbon” because it does not have the maximum number of hydrogen atoms • All alkynes end in - yne • Prefixes are the same as alkanes and ...
Article Summaries
... One of the major downsides to the original Shilov reaction is the fact that it uses platinum consumptively. The goal of this set of experiments was to determine if a non-consumptive pathway was possible. The scorpionate ligands Tp’ (hydrotris(3,5-dimethylpyrazolyl)borate) and Tp (hydridotris(pyrazol ...
... One of the major downsides to the original Shilov reaction is the fact that it uses platinum consumptively. The goal of this set of experiments was to determine if a non-consumptive pathway was possible. The scorpionate ligands Tp’ (hydrotris(3,5-dimethylpyrazolyl)borate) and Tp (hydridotris(pyrazol ...
Abstracts - Thieme Verlag
... This chapter provides a concise overview of metal-catalyzed additions to alkenes that involve carbon monoxide and another nucleophilic species, such as water or an alcohol. This is an important area of research in terms of several commodity chemical targets, with many papers devoted to the evolution ...
... This chapter provides a concise overview of metal-catalyzed additions to alkenes that involve carbon monoxide and another nucleophilic species, such as water or an alcohol. This is an important area of research in terms of several commodity chemical targets, with many papers devoted to the evolution ...
Carbon Chemistry
... Simple sugar formula – C6H12O6 Exist as straight chains or rings Carbohydrates are made up of simple sugars, more complex sugars, and polymers. ...
... Simple sugar formula – C6H12O6 Exist as straight chains or rings Carbohydrates are made up of simple sugars, more complex sugars, and polymers. ...
South Pasadena · AP Chemistry
... C2H6(g) + O2(g) CO2(g) + H2O(l) the following suggestions were made: C2H6(g) + 5O2(g) 2CO2(g) + 3H2O(l) C2H6(g) + 5O(g) 2CO(g) + 3H2O(l) 2C2H6(g) + 7O2(g) 4CO2(g) + 6H2O(l) Which answer is correct and what is wrong with the others? ...
... C2H6(g) + O2(g) CO2(g) + H2O(l) the following suggestions were made: C2H6(g) + 5O2(g) 2CO2(g) + 3H2O(l) C2H6(g) + 5O(g) 2CO(g) + 3H2O(l) 2C2H6(g) + 7O2(g) 4CO2(g) + 6H2O(l) Which answer is correct and what is wrong with the others? ...
oxidation and reduction
... d) Titanium dissolves in concentrated hydrochloric acid to give titanium(III) chloride and hydrogen. Construct an ionic equation for this reaction by writing down two ionic half-equations and then combining them. ...
... d) Titanium dissolves in concentrated hydrochloric acid to give titanium(III) chloride and hydrogen. Construct an ionic equation for this reaction by writing down two ionic half-equations and then combining them. ...
Exam 1 Review Sheet Chapter 15 Chemistry 110b
... functional groups. Know the mechanism of formation/hydrolysis for an acetal (e.g., acetaldehyde + 2CH3OH + H+(cat.)). Be aware of the utility of acetals and ketals [and their thio (sulfur) analogs] as protecting groups in organic synthesis. [10e, 744-750; 9e, 699-705] ...
... functional groups. Know the mechanism of formation/hydrolysis for an acetal (e.g., acetaldehyde + 2CH3OH + H+(cat.)). Be aware of the utility of acetals and ketals [and their thio (sulfur) analogs] as protecting groups in organic synthesis. [10e, 744-750; 9e, 699-705] ...
CHAPTER 10 Properties and Preparation of Alcohols
... Synthesis of Alcohols (Review) • Nucleophilic substitution (usually SN2) of alkyl halide. • Alkene Addition: – Water in acid solution (suffers from rearrangements). – Oxymercuration–demercuration. – Hydroboration–oxidation. ...
... Synthesis of Alcohols (Review) • Nucleophilic substitution (usually SN2) of alkyl halide. • Alkene Addition: – Water in acid solution (suffers from rearrangements). – Oxymercuration–demercuration. – Hydroboration–oxidation. ...
evans enolate alkylation
... (superficially) a surprise here. Firstly, because the oxazolidone is essentially an amide and of significant size, it is once again impossible to get anything other than the Z- enolate. The key is that even though there is a chelate in the boron enolate, when the aldol occur the chelate must fall ap ...
... (superficially) a surprise here. Firstly, because the oxazolidone is essentially an amide and of significant size, it is once again impossible to get anything other than the Z- enolate. The key is that even though there is a chelate in the boron enolate, when the aldol occur the chelate must fall ap ...
A Facile and Environmentally Friendly Disposal of Sodium and
... residues are placed on the dry sand. On top of the sodium a further layer of dry sand is placed. Finally the flower pot is placed in a large porcelain tray or dish. Water is added into the dish. After a few minutes water is drawn by capillary network into the sand. After 1-2 days all sodium is conve ...
... residues are placed on the dry sand. On top of the sodium a further layer of dry sand is placed. Finally the flower pot is placed in a large porcelain tray or dish. Water is added into the dish. After a few minutes water is drawn by capillary network into the sand. After 1-2 days all sodium is conve ...
organic chemistry i
... Addition of hydrogen halides. Markovnikov's rule. Regioselective reactions Addition of hydrogen bromide. Peroxide effect Addition of sulfuric acid Addition of water. Hydration Addition of alkanes. Alkylation Ozonolysis Analysis of alkenes ...
... Addition of hydrogen halides. Markovnikov's rule. Regioselective reactions Addition of hydrogen bromide. Peroxide effect Addition of sulfuric acid Addition of water. Hydration Addition of alkanes. Alkylation Ozonolysis Analysis of alkenes ...
Ch 21 Carboxylic Acid Derivatives
... Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst’n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace “ic acid” with “yl halide”, such as propionyl chloride (a common name) and propanoyl bromide (a systematic name). Replace “carboxylic acid” with “carbonyl hali ...
... Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst’n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace “ic acid” with “yl halide”, such as propionyl chloride (a common name) and propanoyl bromide (a systematic name). Replace “carboxylic acid” with “carbonyl hali ...
Annexure `CD-01` L T P/S SW/FW TOTAL CREDIT UNITS 3 1 4 0 6
... Amines: Structure and nomenclature of amines, physical properties. Separation of a mixture of primary, secondary and tertiary amines. Structural features affecting basicity of amines. Preparation of alkyl and aryl amines (reduction of nitro compounds, nitriles, reductive amination of aldehydic and ...
... Amines: Structure and nomenclature of amines, physical properties. Separation of a mixture of primary, secondary and tertiary amines. Structural features affecting basicity of amines. Preparation of alkyl and aryl amines (reduction of nitro compounds, nitriles, reductive amination of aldehydic and ...
CHEMISTRY SAMPLE PAPER - I
... (iii) what type of hybridisation will Mn+ ion have? (iv) name the type of isomerism exhibited by this complex. 2 14. A mixed oxide of iron and chromium FeOCr2O3 is fused with sodium carbonate in the presence of air to form a yellow coloured compound (A). On acidification the compound (A) forms an or ...
... (iii) what type of hybridisation will Mn+ ion have? (iv) name the type of isomerism exhibited by this complex. 2 14. A mixed oxide of iron and chromium FeOCr2O3 is fused with sodium carbonate in the presence of air to form a yellow coloured compound (A). On acidification the compound (A) forms an or ...
CH1710 PrEX#2 Sp2013 answers
... 1. Aqueous copper (II) chloride reacts with aqueous sodium hydroxide to yield solid copper (II) hydroxide and aqueous sodium chloride. ...
... 1. Aqueous copper (II) chloride reacts with aqueous sodium hydroxide to yield solid copper (II) hydroxide and aqueous sodium chloride. ...
4 H rev quest unit 2
... When ozone, O3, is bubbled into a solution of a straight-chain alkene, an ozonide is formed. This compound decomposes on treatment with water. The reaction can be represented: O3 ...
... When ozone, O3, is bubbled into a solution of a straight-chain alkene, an ozonide is formed. This compound decomposes on treatment with water. The reaction can be represented: O3 ...
(a) Draw a primary, a secondary, and a tertiary alcohol for the
... product is CH3CH2CH2COOH. The functional group in the product is carboxylic acid. Reaction with concentrated H2SO4 is an elimination reaction. A hydrogen atom and the -OH group on (adjacent) carbon atoms are removed forming a (carbon-to-carbon) double bond. The product is CH3CH2CH=CH2. The functiona ...
... product is CH3CH2CH2COOH. The functional group in the product is carboxylic acid. Reaction with concentrated H2SO4 is an elimination reaction. A hydrogen atom and the -OH group on (adjacent) carbon atoms are removed forming a (carbon-to-carbon) double bond. The product is CH3CH2CH=CH2. The functiona ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.