CHEM 201 Name Quiz 10 (Ch 17) ID Q1. Which of the following
... b) reaction with PBr3 followed by NaCl c) reaction with tosyl followed by hydroxide ...
... b) reaction with PBr3 followed by NaCl c) reaction with tosyl followed by hydroxide ...
Blank Final Exam from 2004 - Department of Chemistry | Oregon
... Iron is being reduced and Sulfur is being oxidized Hydrogen is being reduced and Iron is being oxidized Sulfur is being reduced and iron is being oxidized Sulfur is the oxidizing agent and iron is the reducing agent Hydrogen is being oxidized and sulfur is being reduced ...
... Iron is being reduced and Sulfur is being oxidized Hydrogen is being reduced and Iron is being oxidized Sulfur is being reduced and iron is being oxidized Sulfur is the oxidizing agent and iron is the reducing agent Hydrogen is being oxidized and sulfur is being reduced ...
Enantiospecific skeleton expanding cross
... cost organic synthesis. In just one example, a lactic acid derivative is converted to 2-methylhexanoic acid with excellent yield. This compound is an important building block in the production of the potential high-potency sweetener NC-00637. Ultimately, this new reaction presents an extremely attra ...
... cost organic synthesis. In just one example, a lactic acid derivative is converted to 2-methylhexanoic acid with excellent yield. This compound is an important building block in the production of the potential high-potency sweetener NC-00637. Ultimately, this new reaction presents an extremely attra ...
اســـم المـــدرس: د
... ] Oxidation of ketone to alcohol is faster than the oxidation of aldehydes ...
... ] Oxidation of ketone to alcohol is faster than the oxidation of aldehydes ...
AMINO ACIDS Ethan Secor, John N. Gitua (Mentor)
... and an amine group. When these groups are attached to the same carbon atom the compound is known as an -amino acid. ...
... and an amine group. When these groups are attached to the same carbon atom the compound is known as an -amino acid. ...
Slide 1
... This is a nucleophilic addition–elimination reaction The pH of the reaction must be controlled ...
... This is a nucleophilic addition–elimination reaction The pH of the reaction must be controlled ...
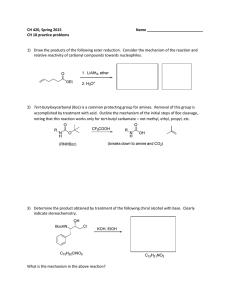
CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
... 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ...
... 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ...
Practical, Asymmetric Redox-Neutral Chemical Synthesis via Borrowing Hydrogen
... available alcohols through a multi-step process involving oxidation of alcohol to ketone, condensation with amine to imine followed by reduction. As both the oxidation and reduction steps in this process utilize stoichiometric reagents, much waste is generated. The concept of “borrowing hydrogen” ha ...
... available alcohols through a multi-step process involving oxidation of alcohol to ketone, condensation with amine to imine followed by reduction. As both the oxidation and reduction steps in this process utilize stoichiometric reagents, much waste is generated. The concept of “borrowing hydrogen” ha ...
reactions of the carbonyl group in aldehydes and ketones
... A curly arrow is a symbol used in reaction mechanisms to show the movement of an electron pair in the braking or forming of a covalent bond ...
... A curly arrow is a symbol used in reaction mechanisms to show the movement of an electron pair in the braking or forming of a covalent bond ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.