Exam 4
... 3) The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? a) Some hydrogen atoms are heavier than others b) The difference is the binding energy of the helium nucleus c) The difference is the experimental ...
... 3) The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? a) Some hydrogen atoms are heavier than others b) The difference is the binding energy of the helium nucleus c) The difference is the experimental ...
Biochemistry Carbon Compounds Supplement 1 Name: . Answer
... 4. What role do functional groups play in the molecules in which they are found? 5. How are monomers, polymers, and macromolecules related to each other? 6. How is a polymer broken down? 7. Why is ATP referred to as the “energy currency” in living things? 8. Analyzing concepts Humans are about 65% w ...
... 4. What role do functional groups play in the molecules in which they are found? 5. How are monomers, polymers, and macromolecules related to each other? 6. How is a polymer broken down? 7. Why is ATP referred to as the “energy currency” in living things? 8. Analyzing concepts Humans are about 65% w ...
8. Chemistry of cooking
... Propanone is a widely used solvent. It can be made from propene. Using full structural formulae show the steps involved in this preparation and name the reagent used in each step. ...
... Propanone is a widely used solvent. It can be made from propene. Using full structural formulae show the steps involved in this preparation and name the reagent used in each step. ...
CHE 322
... 3. (8) Give the complete mechanism that shows why the reaction of butanal with a cyclic 2° amine followed by heating with acid produces an enamine that is nucleophilic at butanal’s former α-carbon. ...
... 3. (8) Give the complete mechanism that shows why the reaction of butanal with a cyclic 2° amine followed by heating with acid produces an enamine that is nucleophilic at butanal’s former α-carbon. ...
$doc.title
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS
... APPLICATION TO THE ENANTIOSELECTIVE HENRY REACTION Doctoral Thesis by Victor Hernández Olmos The synthesis of new molecules that can work as chiral ligands in metal catalyzed enantioselective reactions is currently an area of great interest.1,2 These ligands must be capable of coordinating with the ...
... APPLICATION TO THE ENANTIOSELECTIVE HENRY REACTION Doctoral Thesis by Victor Hernández Olmos The synthesis of new molecules that can work as chiral ligands in metal catalyzed enantioselective reactions is currently an area of great interest.1,2 These ligands must be capable of coordinating with the ...
Document
... (A. DNP Test for Carbonyl Compound) The reaction of an aldehyde or ketone with 2,4-dinitrophenylhydrazin yields a precipitate of a 2,4- dinitrophenylhydrazin, or DNP. Alcohols and phenols do not undergo reaction with this reagent. (B. Ceric Nitrate Test for Alcohols and Phenols) Alcohols or phenols ...
... (A. DNP Test for Carbonyl Compound) The reaction of an aldehyde or ketone with 2,4-dinitrophenylhydrazin yields a precipitate of a 2,4- dinitrophenylhydrazin, or DNP. Alcohols and phenols do not undergo reaction with this reagent. (B. Ceric Nitrate Test for Alcohols and Phenols) Alcohols or phenols ...
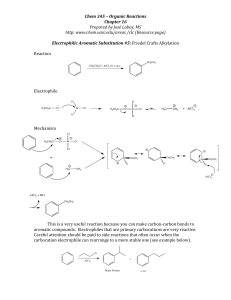
EAS Friedel-Crafts Alkylation
... This is a very useful reaction because you can make carbon-‐carbon bonds to aromatic compounds. Electrophiles that are primary carbocations are very reactive. Careful attention should be paid to side ...
... This is a very useful reaction because you can make carbon-‐carbon bonds to aromatic compounds. Electrophiles that are primary carbocations are very reactive. Careful attention should be paid to side ...
What are reactions?
... 1. Hydrogen and carbon dioxide are __________. If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical cha ...
... 1. Hydrogen and carbon dioxide are __________. If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical cha ...
What are reactions? - UTLNET Secure Site
... 1. Hydrogen and carbon dioxide are __________. If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical cha ...
... 1. Hydrogen and carbon dioxide are __________. If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical cha ...
Unit 3 Goals - kimscience.com
... o complete and balance combustion reactions of organic molecules containing carbon, hydrogen and oxygen, and explain the reaction in terms of bonds breaking and forming, enthalpy, and entropy change. o distinguish between complete and incomplete combustion in terms of reaction conditions, resulting ...
... o complete and balance combustion reactions of organic molecules containing carbon, hydrogen and oxygen, and explain the reaction in terms of bonds breaking and forming, enthalpy, and entropy change. o distinguish between complete and incomplete combustion in terms of reaction conditions, resulting ...
1. Rank the following compounds in order of decreasing acidity (1
... 2. Show the enol tautomer of 1,3,5-cyclohexatrione. Would you expect this compound to exist predominantly in the keto or enol form? O ...
... 2. Show the enol tautomer of 1,3,5-cyclohexatrione. Would you expect this compound to exist predominantly in the keto or enol form? O ...
doc CHEM 222 Lab exam with Answers
... 14._T___ The Horner-Wadsworth-Emmons reaction (modification of a Wittig) performed in the lab made use of “instant ylides”. These are solid phase mixtures of a phosphonium salt with sodium amide. This question was eliminated 15.___ F___A cloudy solution is often wet but a clear solution is always dr ...
... 14._T___ The Horner-Wadsworth-Emmons reaction (modification of a Wittig) performed in the lab made use of “instant ylides”. These are solid phase mixtures of a phosphonium salt with sodium amide. This question was eliminated 15.___ F___A cloudy solution is often wet but a clear solution is always dr ...
Microsoft Word
... inversion of the stereocenter at C-3 of the mesylate giving methyl-2-acetamido4,6-O-benzylidene-2-deoxy-a-D-ribo-hexopyranoside 13, which was confirmed by the spectral studies. The hydroxyl group of the compound 13 was protected as its methyl ether 14 using NaH and methyl iodide. Figure The regiosel ...
... inversion of the stereocenter at C-3 of the mesylate giving methyl-2-acetamido4,6-O-benzylidene-2-deoxy-a-D-ribo-hexopyranoside 13, which was confirmed by the spectral studies. The hydroxyl group of the compound 13 was protected as its methyl ether 14 using NaH and methyl iodide. Figure The regiosel ...
AS Chemistry - Module 1 Definitions
... reaction between two different types of molecule to give a polymer accompanied by the loss of a small molecule such as water ...
... reaction between two different types of molecule to give a polymer accompanied by the loss of a small molecule such as water ...
Worksheet 1 - Oregon State chemistry
... Addition is to make an addition to a molecule. An example is the addition of a small molecule (such as Br2) to an alkene. ...
... Addition is to make an addition to a molecule. An example is the addition of a small molecule (such as Br2) to an alkene. ...
Final Exam from 2006 - Department of Chemistry | Oregon State
... CH3CH3 CH3OH an acetal a hemiacetal an alkene ...
... CH3CH3 CH3OH an acetal a hemiacetal an alkene ...
Study Guide on Ch 5 and 6
... F. What is a hemiacetal group (p. 228) G. What is an anomeric carbon (pp. 228 – 229) a. What are alpha- () and beta- () anomers? (p. 229 bottom) H. Primary alcohols Oxidize to corresponding aldehydes (see the equation below) I. Aldehydes are further oxidized to carboxylic acids. J. Secondary alcoh ...
... F. What is a hemiacetal group (p. 228) G. What is an anomeric carbon (pp. 228 – 229) a. What are alpha- () and beta- () anomers? (p. 229 bottom) H. Primary alcohols Oxidize to corresponding aldehydes (see the equation below) I. Aldehydes are further oxidized to carboxylic acids. J. Secondary alcoh ...
Synthesis, Isolation and Purification of an Ester
... the general outline for the procedure (refluxing, extraction, and distillation) and ask them to calculate the amounts of ethyl alcohol and glacial (17.4 M) acetic acid needed to prepare 15 g of ethyl acetate. Discuss the following prerequisite knowledge: (1) Synthesis of an ester is generally revers ...
... the general outline for the procedure (refluxing, extraction, and distillation) and ask them to calculate the amounts of ethyl alcohol and glacial (17.4 M) acetic acid needed to prepare 15 g of ethyl acetate. Discuss the following prerequisite knowledge: (1) Synthesis of an ester is generally revers ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.