Chemistry and the material world
... ΔG = ΔH° -TΔS° The sign of ΔG tells us the direction in which a reaction will proceed to reach an equilibrium and the magnitude of ΔG tells us how far from equilibrium the reaction still is (ΔG = 0 means equilibrium). Thus, as the reaction progresses, the magnitude of ΔG will become smaller and sma ...
... ΔG = ΔH° -TΔS° The sign of ΔG tells us the direction in which a reaction will proceed to reach an equilibrium and the magnitude of ΔG tells us how far from equilibrium the reaction still is (ΔG = 0 means equilibrium). Thus, as the reaction progresses, the magnitude of ΔG will become smaller and sma ...
212Final`97
... 4. (12) State whether further electrophilic substitution of each of the following molecules would occur in the ortho / para or meta positions. It is not necessary to explain why. O O H a) b) c) d) S N Br N NH2 CH3 ...
... 4. (12) State whether further electrophilic substitution of each of the following molecules would occur in the ortho / para or meta positions. It is not necessary to explain why. O O H a) b) c) d) S N Br N NH2 CH3 ...
An Efficient Method for Selective Deprotection of Trimethylsilyl
... reagents on various inorganic surfaces have recently attracted attention. The most advantage of these methods over conventional classical method is that they show cleaner reactions, decreased reaction time and straightforward workup. In continuation of our ongoing program to develop environmentally ...
... reagents on various inorganic surfaces have recently attracted attention. The most advantage of these methods over conventional classical method is that they show cleaner reactions, decreased reaction time and straightforward workup. In continuation of our ongoing program to develop environmentally ...
Mr. Hauptman Organic Chemistry
... This compound is classified as (1) an aldehyde (3) an amine (2) an amide (4) a ketone 32) Given the structural formula: This structural formula represents a molecule of (1) an aldehyde (3) a ketone (2) an ester (4) an amino acid Base your answers to questions 33 and 34 on the information below. Give ...
... This compound is classified as (1) an aldehyde (3) an amine (2) an amide (4) a ketone 32) Given the structural formula: This structural formula represents a molecule of (1) an aldehyde (3) a ketone (2) an ester (4) an amino acid Base your answers to questions 33 and 34 on the information below. Give ...
Organic Chemistry Fifth Edition
... Substitutive names start with the longest contiguous carbon chain that bears the –OH group. Number from the side closest to the OH group and replace the –ane of the corresponding alkane with –ol. List substituents and their locants before the parent name. ...
... Substitutive names start with the longest contiguous carbon chain that bears the –OH group. Number from the side closest to the OH group and replace the –ane of the corresponding alkane with –ol. List substituents and their locants before the parent name. ...
Final Review 2006
... c. disassociates into ions. b. forms a precipitate. d. reacts with the water. ...
... c. disassociates into ions. b. forms a precipitate. d. reacts with the water. ...
PPT - Unit 5
... 2. Given the following data: -(C2H2(g) + 5/2O2(g) → 2CO2(g) + H2O(l) ΔH = -1300. kJ) 2( C(s) + O2(g) → CO2(g) ) 2(ΔH = -394 kJ) H2(g) + 1/2O2(g) → H2O(l) ΔH = -286 kJ Calculate ΔH for the following reaction: 2C(s) + H2(g) → C2H2(g) 2C(s) + 2O2(g) → 2CO2(g) ΔH = -788 kJ 2CO2(g) + H2O(l) → C2H2(g) + ...
... 2. Given the following data: -(C2H2(g) + 5/2O2(g) → 2CO2(g) + H2O(l) ΔH = -1300. kJ) 2( C(s) + O2(g) → CO2(g) ) 2(ΔH = -394 kJ) H2(g) + 1/2O2(g) → H2O(l) ΔH = -286 kJ Calculate ΔH for the following reaction: 2C(s) + H2(g) → C2H2(g) 2C(s) + 2O2(g) → 2CO2(g) ΔH = -788 kJ 2CO2(g) + H2O(l) → C2H2(g) + ...
Functional Groups 2
... H-bond w/ each other -Can not H-bond between each other but can H-bond to solvents like water - Lower MW compounds are appreciably soluble in water ...
... H-bond w/ each other -Can not H-bond between each other but can H-bond to solvents like water - Lower MW compounds are appreciably soluble in water ...
Chapter 10 - UCSB CLAS
... IV. Arene Oxides ⇒ In this chapter you will see arene oxides undergoing either nucleophilic substitution reactions same as epoxides in basic/neutral conditions or rearrangement reactions to produce phenol (primary focus) – since there’s a carbocation formed in the rearrangement reaction this will de ...
... IV. Arene Oxides ⇒ In this chapter you will see arene oxides undergoing either nucleophilic substitution reactions same as epoxides in basic/neutral conditions or rearrangement reactions to produce phenol (primary focus) – since there’s a carbocation formed in the rearrangement reaction this will de ...
Macromolecules Basic Facts: Most are polymers – large molecules
... been converted to saturated fats; peanut butter and margarine ...
... been converted to saturated fats; peanut butter and margarine ...
6CH02 - MPPE
... SECTION A Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question, select one answer from A to D and put a cross in the box . and then mark your new answer with If you change your mind, put a line through the box a cross . 1 The c ...
... SECTION A Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question, select one answer from A to D and put a cross in the box . and then mark your new answer with If you change your mind, put a line through the box a cross . 1 The c ...
C:\Users\mrh70950\Documents\My Files\WordPerfect
... I. 50:50 H2SO4:H2O; Hg+2 usually is added II. Mechanism is tricky, as it involves a rearrangement of the enol product to a thermodynamically more stable carbonyl-containing product III. Process is called tautomerization ii. antiMarkovnikov I. Borohydration-oxidation d. dihalogenation: addition of X2 ...
... I. 50:50 H2SO4:H2O; Hg+2 usually is added II. Mechanism is tricky, as it involves a rearrangement of the enol product to a thermodynamically more stable carbonyl-containing product III. Process is called tautomerization ii. antiMarkovnikov I. Borohydration-oxidation d. dihalogenation: addition of X2 ...
Paper
... (b) From July 2008 changes will apply to the way in which taxes are levied on new cars bought in Ireland. Vehicles that, in controlled tests, have higher levels of carbon dioxide emission per kilometre travelled will be subject to higher levels of taxation. The measures are designed to encourage the ...
... (b) From July 2008 changes will apply to the way in which taxes are levied on new cars bought in Ireland. Vehicles that, in controlled tests, have higher levels of carbon dioxide emission per kilometre travelled will be subject to higher levels of taxation. The measures are designed to encourage the ...
Kinetic studies on the oxidation of cyclohexanone by potassium
... carboxylic acids etc. can be produced by the oxidation of related substrates by various oxidizing agents (Lee, 1980; Wiberg, 1965). Permanganate, chromate, hypochlorite etc. are extensively used for the primary and secondary alcohol oxidation reactions in a selective manner to get corresponding alde ...
... carboxylic acids etc. can be produced by the oxidation of related substrates by various oxidizing agents (Lee, 1980; Wiberg, 1965). Permanganate, chromate, hypochlorite etc. are extensively used for the primary and secondary alcohol oxidation reactions in a selective manner to get corresponding alde ...
Topic: Functional groups
... Topic: Functional groups Do Now: All of the following are Hydrocarbons. Why are some gases at room temp. while others are liquid at room temp.? Why are none solids at room temp.? ...
... Topic: Functional groups Do Now: All of the following are Hydrocarbons. Why are some gases at room temp. while others are liquid at room temp.? Why are none solids at room temp.? ...
Midterm 2 from Summer 2012
... Hydrogen Cyanide, HCN, can be made in a two step process. First, Ammonia is reacted with Oxygen to give Nitrogen Oxide: 4 NH3(g) + 5 O2(g) ...
... Hydrogen Cyanide, HCN, can be made in a two step process. First, Ammonia is reacted with Oxygen to give Nitrogen Oxide: 4 NH3(g) + 5 O2(g) ...
AP Biology Functional Groups of Carbon
... • Formaldehyde, a preservative • Acetaldehyde, a liver product • Glucose, a combination of aldehyde & alcohol ...
... • Formaldehyde, a preservative • Acetaldehyde, a liver product • Glucose, a combination of aldehyde & alcohol ...
No Slide Title
... 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation ...
... 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation ...
Chapter 4 - Reactions in Aqueous Solutions
... 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation ...
... 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation ...
Reactions of 2, 6-cycloheptadienone and 2, 7
... aldehyde and acetonedicarboxylic acid, Robinson' also noted '(that tropinone might result.. .by the addition of methylamine to a cycloheptadienone. . . . I ' Yearly 10 years ago, Hor&k2reported the characterization by paper chromatography of tropinone prepared from a large excess of methylamine and ...
... aldehyde and acetonedicarboxylic acid, Robinson' also noted '(that tropinone might result.. .by the addition of methylamine to a cycloheptadienone. . . . I ' Yearly 10 years ago, Hor&k2reported the characterization by paper chromatography of tropinone prepared from a large excess of methylamine and ...
5.2. Related mechanisms of halogen chemistry A large variety of
... While no terminal unsaturated bonds are available by the chosen precursors, these reactions can also occur at other unsaturated bonds of the reactive SOA or at aromatic systems. αpinene induced SOA is suspected to be rather poor on unsaturated bonds. SOA from catechol and guaiacol still exhibits a l ...
... While no terminal unsaturated bonds are available by the chosen precursors, these reactions can also occur at other unsaturated bonds of the reactive SOA or at aromatic systems. αpinene induced SOA is suspected to be rather poor on unsaturated bonds. SOA from catechol and guaiacol still exhibits a l ...
Introduction to Chemical Equations
... using the names of the reactants and products. Write the word equation for the reaction of methane gas with oxygen gas to form carbon dioxide and water. ...
... using the names of the reactants and products. Write the word equation for the reaction of methane gas with oxygen gas to form carbon dioxide and water. ...
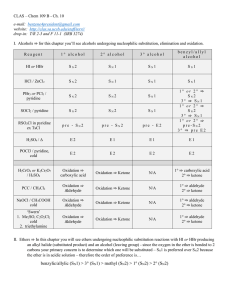
12.1 Alcohols: Structure and Physical Properties
... • Dehydration is a type of elimination reaction – A molecule loses atoms or ions from its structure – Here –OH and –H are removed / eliminate from adjacent carbon atoms to produce an alkene and water – A reversal of the hydration reaction that forms alcohols ...
... • Dehydration is a type of elimination reaction – A molecule loses atoms or ions from its structure – Here –OH and –H are removed / eliminate from adjacent carbon atoms to produce an alkene and water – A reversal of the hydration reaction that forms alcohols ...
C4C5C6
... Ozone filters out and stops harmful ultraviolet light from reaching the surface of the earth CFCs were used as refrigerants and in aerosols because they have a low boiling point, are insoluble in water and are very unreactive. Use of CFCs in the UK is now banned to stop any more damage to the ozone ...
... Ozone filters out and stops harmful ultraviolet light from reaching the surface of the earth CFCs were used as refrigerants and in aerosols because they have a low boiling point, are insoluble in water and are very unreactive. Use of CFCs in the UK is now banned to stop any more damage to the ozone ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.