CHEMISTRY 1000
... hydrogen to a carbonyl (using NaBH4 or LiAlH4 as the source of nucleophilic hydrogen). This was a chemoselective reaction – in other words, the reducing agent only reduced one functional group (the carbonyl) and left others alone (e.g. alkenes). If we want to reduce an alkene or alkyne, we need to u ...
... hydrogen to a carbonyl (using NaBH4 or LiAlH4 as the source of nucleophilic hydrogen). This was a chemoselective reaction – in other words, the reducing agent only reduced one functional group (the carbonyl) and left others alone (e.g. alkenes). If we want to reduce an alkene or alkyne, we need to u ...
A Model for Catalytically Active Zinc(I1) Ion in Liver
... (11). Identification of reaction products was performed by coinjection of reaction mixtures with standards onto HPLC column and/or by IH N M R measurements of isolated products. HPLC analysis was performed by a 5 pm Lichrospher Si60 column (250 X $50 mm) eluted with 1:l n-hexanelethyl acetate at 1.5 ...
... (11). Identification of reaction products was performed by coinjection of reaction mixtures with standards onto HPLC column and/or by IH N M R measurements of isolated products. HPLC analysis was performed by a 5 pm Lichrospher Si60 column (250 X $50 mm) eluted with 1:l n-hexanelethyl acetate at 1.5 ...
answer
... Priorities at *2: –NHCOR > –COOH > –(CH2)4NH2 > –H With H at front these groups go clockwise. Therefore, with H at back, they would go anticlockwise. Therefore (S)- configuration about *2. Give the products when molecule (M) is hydrolysed by heating it with 6 M HCl. Make sure you show the products i ...
... Priorities at *2: –NHCOR > –COOH > –(CH2)4NH2 > –H With H at front these groups go clockwise. Therefore, with H at back, they would go anticlockwise. Therefore (S)- configuration about *2. Give the products when molecule (M) is hydrolysed by heating it with 6 M HCl. Make sure you show the products i ...
Fundamentals Of Organic Chemistry
... Mechanism of the dehydration of alcohol is discussed below, and also in elimination reactions, later in this book. Mechanism Protonation of the hydroxyl group of the alcohol followed by the loss of water molecule to give a 1° carbocation. The 1° carbocation undergo rearrangement to form more stable ...
... Mechanism of the dehydration of alcohol is discussed below, and also in elimination reactions, later in this book. Mechanism Protonation of the hydroxyl group of the alcohol followed by the loss of water molecule to give a 1° carbocation. The 1° carbocation undergo rearrangement to form more stable ...
FREE Sample Here
... 11) Ionic bonds are formed when A) atoms share electrons. B) two or more atoms lose electrons at the same time. C) electrons are completely transferred from one atom to another. D) hydrogen forms bonds with negatively charged atoms in the same or different molecule. E) a pair of electrons is shared ...
... 11) Ionic bonds are formed when A) atoms share electrons. B) two or more atoms lose electrons at the same time. C) electrons are completely transferred from one atom to another. D) hydrogen forms bonds with negatively charged atoms in the same or different molecule. E) a pair of electrons is shared ...
Esters A class of organic compounds that react with water to
... links the carbon atoms together. If there is a separation of a continuous link of carbon atoms due to the oxygen atom, individually name the two alkanes before and after the oxygen atom. The longer the structural alkane is the one that should contain the carbonyl group. The format is as folows: ...
... links the carbon atoms together. If there is a separation of a continuous link of carbon atoms due to the oxygen atom, individually name the two alkanes before and after the oxygen atom. The longer the structural alkane is the one that should contain the carbonyl group. The format is as folows: ...
Section (c) – The Structure of atoms
... used in catalytic converters. In the catalytic converter harmful carbon monoxide and oxides of nitrogen are converted into less harmful .................................... ......................................... and nitrogen. 7. A homogeneous catalyst is in the ............................ physic ...
... used in catalytic converters. In the catalytic converter harmful carbon monoxide and oxides of nitrogen are converted into less harmful .................................... ......................................... and nitrogen. 7. A homogeneous catalyst is in the ............................ physic ...
Chemical Reaction and Matter Review
... information that it provides will vary slightly. Before we go about learning how to write chemical formulas, it is important that you clearly understand the difference between covalent (molecular) compounds and ionic compounds. Ionic compounds are composed of charged ions that are held together by e ...
... information that it provides will vary slightly. Before we go about learning how to write chemical formulas, it is important that you clearly understand the difference between covalent (molecular) compounds and ionic compounds. Ionic compounds are composed of charged ions that are held together by e ...
1st Olympiad of Metropolises Chemistry Theoretical Problems
... Moscow State University Yu. K. Yuriev developed industrial transformation of furans into pyrroles under heating of furan with ammonia (amines) above 400 C in the presence of alumina. In a laboratory, the sequence of furan hydrolysis followed by Paal-Knorr reaction with ammonia (amine) is used for t ...
... Moscow State University Yu. K. Yuriev developed industrial transformation of furans into pyrroles under heating of furan with ammonia (amines) above 400 C in the presence of alumina. In a laboratory, the sequence of furan hydrolysis followed by Paal-Knorr reaction with ammonia (amine) is used for t ...
The 9-Phenyl-9-fluorenyl Group for Nitrogen Protection in
... the stability in basic conditions. In strongly basic conditions the Pf anion can act as a leaving group giving rise to an imine (Scheme 1). This elimination occurs faster/easier than the inversion and reprotonation of the anion [5]. Scheme 1. The decomposition of N-Pf amino aldehyde in basic conditi ...
... the stability in basic conditions. In strongly basic conditions the Pf anion can act as a leaving group giving rise to an imine (Scheme 1). This elimination occurs faster/easier than the inversion and reprotonation of the anion [5]. Scheme 1. The decomposition of N-Pf amino aldehyde in basic conditi ...
Chapter 4 (Hill/Petrucci/McCreary/Perry Chemical Reactions in
... In addition to acid-base and precipitation reactions, there is a third type of reaction: oxidationreduction or redox reactions. Oxidation-reduction reactions are electron exchange reactions (electron = e1-) Oxidation - loss of 1 or more electrons by an ion or molecule Reduction - gain of 1 or more e ...
... In addition to acid-base and precipitation reactions, there is a third type of reaction: oxidationreduction or redox reactions. Oxidation-reduction reactions are electron exchange reactions (electron = e1-) Oxidation - loss of 1 or more electrons by an ion or molecule Reduction - gain of 1 or more e ...
Period 5
... • When a hydroxyl group is substituted for one hydrogen atom in ethane, the result alcohol is ethanol (C2H5OH). • Ethanol is also added to gasoline to make fuel. • Ethanol is produced naturally by yeast, or bacteria in corn wheat, and barley. • It’s also used in medicine and in alcoholic drinks. • W ...
... • When a hydroxyl group is substituted for one hydrogen atom in ethane, the result alcohol is ethanol (C2H5OH). • Ethanol is also added to gasoline to make fuel. • Ethanol is produced naturally by yeast, or bacteria in corn wheat, and barley. • It’s also used in medicine and in alcoholic drinks. • W ...
Methanol Synthesis
... Since the experimental data are all at constant temperature, the Activation Energies are kept fixed at zero. Only the pre-exponentials factors for the two reactions that produce methanol will be estimated. Another issue is that the methanol measurement in the reactor effluent is provided as a molar ...
... Since the experimental data are all at constant temperature, the Activation Energies are kept fixed at zero. Only the pre-exponentials factors for the two reactions that produce methanol will be estimated. Another issue is that the methanol measurement in the reactor effluent is provided as a molar ...
organic chemistry - Turner Fenton Secondary School
... is done such that the alkyl groups or other substituents have the lowest possible number. ...
... is done such that the alkyl groups or other substituents have the lowest possible number. ...
Chapter 16_CHEM 131
... hydrogen attached to the nitrogen that can be removed by adding a base. • Quaternary salts are present in solution in only one form, which is independent of the pH of the solution. ...
... hydrogen attached to the nitrogen that can be removed by adding a base. • Quaternary salts are present in solution in only one form, which is independent of the pH of the solution. ...
Chapters 6 and 17: Chemical Thermodynamics
... Know the First and Second laws of thermodynamics Calculate the heat required to heat water in all 3 phases, and between phases Label an energy diagram (exothermic and endothermic) Determine the change in standard enthalpy of a reaction (delta H) Determine the change in standard entropy of ...
... Know the First and Second laws of thermodynamics Calculate the heat required to heat water in all 3 phases, and between phases Label an energy diagram (exothermic and endothermic) Determine the change in standard enthalpy of a reaction (delta H) Determine the change in standard entropy of ...
Acid-Base Reactions
... Mg is oxidized and Co2+ is reduced. 34. Which of the following are oxidation-reduction reactions? (Select all that apply.) 1. (Cr2O7)2- (aq) + 2 (OH)- (aq) ---> 2 (CrO4)2- (aq) + (H2O) (l) 2. HCl(aq) + KOH(aq) ---> KCl(aq) + H2O(l) 3. O3(g) + NO(g) ---> O2(g) + NO2(g) 4. CH4(g) + 2O2(g) ---> CO2(g) ...
... Mg is oxidized and Co2+ is reduced. 34. Which of the following are oxidation-reduction reactions? (Select all that apply.) 1. (Cr2O7)2- (aq) + 2 (OH)- (aq) ---> 2 (CrO4)2- (aq) + (H2O) (l) 2. HCl(aq) + KOH(aq) ---> KCl(aq) + H2O(l) 3. O3(g) + NO(g) ---> O2(g) + NO2(g) 4. CH4(g) + 2O2(g) ---> CO2(g) ...
Question paper - Edexcel
... Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question, select one answer from A to D and put a cross in the box . and then mark your new answer with a If you change your mind, put a line through the box cross . 1 In which of the ...
... Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question, select one answer from A to D and put a cross in the box . and then mark your new answer with a If you change your mind, put a line through the box cross . 1 In which of the ...
Solutions
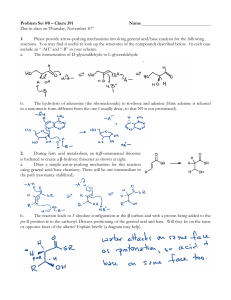
... Please provide arrow-pushing mechanisms involving general acid/base catalysis for the following reactions. You may find it useful to look up the structures of the compounds described below. In each case include an “-AH” and “-B” in your scheme. a. The isomerization of D-glyceraldehyde to L-glycerald ...
... Please provide arrow-pushing mechanisms involving general acid/base catalysis for the following reactions. You may find it useful to look up the structures of the compounds described below. In each case include an “-AH” and “-B” in your scheme. a. The isomerization of D-glyceraldehyde to L-glycerald ...
Document
... • Name the longest chain possible. • As a prefix name the chain attached with – yl on the end and give the number of the carbon atom it is attached to ...
... • Name the longest chain possible. • As a prefix name the chain attached with – yl on the end and give the number of the carbon atom it is attached to ...
Halogens - Cronodon
... The hypochlorous acid produced is a powerful oxidising agent, in which the hypochlorous acid is reduced to Cl–. Q.8. Write an ionic half-equation for the reduction of hypochlorous acid to chloride. ...
... The hypochlorous acid produced is a powerful oxidising agent, in which the hypochlorous acid is reduced to Cl–. Q.8. Write an ionic half-equation for the reduction of hypochlorous acid to chloride. ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.