* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
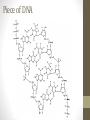
Organic Introduction • Two Group 14 elements, carbon and silicon, form the basis for most natural substances. • Silicon, with its great affinity for oxygen, forms chains and rings containing Si-O-Si bridges to produce silica and silicates that form the basis for most rocks, sands, and soils. Silicon may be the most important element in the geological world. Carbon • Carbon has the unusual ability of bonding to itself to form long chains or rings of carbon atoms. • Carbon forms strong bonds to other nonmetals such as hydrogen, nitrogen, oxygen, sulfur, and the halogens. • Several million (11 million-plus) are known, and the number continues to grow rapidly. • Carbon is the most important compound to the biological world. Organic Chemistry • Organic Chemistry is the study of carboncontaining compounds and their properties. • Oxides and carbonates that contain carbon are not considered to be organic, they are inorganic. • The original distinction between organic and inorganic was based on whether a compound was produced by living things. • Organic chemistry plays a vital role in our quest to understand living systems. • Industrial organic chemistry produces synthetic fibers (nylon, rayon), plastics, rubber (latex) explosives, artificial sweeteners, vinegar and pharmaceuticals that are such an important part of modern life. • The energy on which we rely so heavily on to power our civilization is based mostly on organic materials found in coal and petroleum. Saturated and Unsaturated Hydrocarbons • Hydrocarbons are compounds composed of carbon and hydrogen. • Saturated hydrocarbons contain carbon-carbon bonds that are all single bonds (each carbon is bonded to four atoms). • Unsaturated hydrocarbons contain carbon-carbon multiple bonds and can react with additional atoms. • Saturated means it is “full of hydrogen” • Unsaturated is “missing hydrogens” because of the double/triple bonds. H H H C C H H H H C H C H C C H Copyright © Cengage Learning. All rights reserved Hydrocarbons 7 Root words Meth # of C atoms 1 Hex # of C atoms 6 Eth 2 Hept 7 Prop 3 Oct 8 But 4 Non 9 Pent 5 Dec 10 So for example H HHH H-C-C-C-C-H H HHH butane H HHHHHHH H-C-C-C-C-C-C-C-C-H H HHHHHHH octane H HH H H-C-C-C-H H-C-H H HH H propane methane Molecular Formulas • Alkanes always have the molecular formula of: • CxH2x+2 • 2 H on every C except the end, they get 3 • Hexane• C6H14 molecular formula Lewis Dot, or Structural Formula HHHHHH H-C-C-C-C-C-C-H HHHHHH Skeleton Formulas • Drawing Lewis Dot structural formulas for long organic compounds can get rather tedious. • So organic has shortened it • They don’t write the C’s or the H’s • You draw a jagged line, at each corner there is a Carbon • Assume all extra spaces are filled with H For Example Heptane, C7H16 HHHHHHH H-C-C-C-C-C-C-C-H HHHHHHH = Nonane, C9H20 HHHHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHHHHHH = Isomers • Isomers- compounds with the same molecular formula but different structural formulas • Different structural formulas mean it has different properties • Butane is the first alkane with a possible isomer HHHH = H-C-C-C-C-H HHHH H H H or H-C- C - C-H Both are C H H HCH H H 4 10 Copyright © Cengage Learning. All rights reserved Butane 14 Naming Isomers • Name the longest chain possible. • As a prefix name the chain attached with – yl on the end and give the number of the carbon atom it is attached to 1 6 4 2 3 Longest Chain 5 7 3 ethyl heptane •It could also be 5 ethyl heptane if you started numbering from the other side, when given an option always go with the Lower number!!! Name this molecule And give its molecular formula 4 ethyl octane C10H22 4 propyl decane C13H28 Cyclic Hydrocarbons • A hydrocarbon that is a ring instead of a chain. To name it, give it the prefix “cyclo-” • Molecular Formula • Subtract 2 H from CxH2x+2 • CxH2X • cyclobutane HH H-C-C-H H-C-C-H HH C4H8 Name the following compounds and give their formula cyclohexane cycloheptane C6H12 C7H14 cyclooctane cyclodecane C8H16 C10H20 Name and give the formula Methyl cyclohexane C7H14 Alkenes • Contain a double bond • They get the suffix “-ene” and the number of the carbon atom the double bond is on (lowest number) • Molecular formula • Subtract 2 H for each double bond from Skeleton fomula CxH2x+2 1 butene H H H H-C=C-C-C-H H H H C4H8 Alkynes • Contain a triple bond • They get the suffix “-yne” and the number of the carbon atom the triple bond is on. Molecular formula • subtract 4 H for each triple bond from CxH2x+2 Skeleton fomula H H H H H H 2 pentyne H-C-C=C-C-C-H C5H8 Name and give the formula for these compounds 2 hexene C6H12 Cyclopentane C5H10 3 methyl nonane C10H22 ethyne (commonly known as acetylene) C2H2 3 methyl 1 pentene C6H12 Name and give the formula for these compounds 2 heptene C7H14 Cyclopentene C5H8 cyclopropane C3H6 1 butyne C4H6 3 ethyl 1 hexene C8H16 Doubles and triples • If you have two of the same thing put “di” in front of it • If you have three of the same thing put “tri” in front of it Examples 2,3 hexadiene C6H10 3,4,4 trimethyl heptane C10H22 Multiple groups on a chain • Name each and put them in alphabetical order 3, 4 diethyl 2 methyl 1 heptene C12H24 Common functional groups Functional Groups • Atoms other than hydrogen or carbon covalently bonded to a carbon atom in an organic molecule. • Most commonly oxygen, nitrogen, or the halogens. • The presence of a functional group drastically changes the chemical properties of a molecule. Different Functional groups with a 2 carbon chain • Ethane- gas (found in natural gas) • Ethanol- grain alcohol (drinkable) • Ethanoic acid- vinegar • Diethyl ether- starting fluid • Chloro fluoro ethane (CFC’s used as refrigerants) • Ethanal- foul smelling liquid (similar to formaldehyde) Halogenated Hydrocarbons • Hydrocarbons with halogens attached • Before the main chain name the halogen as either fluoro, chloro, bromo or iodo and give its number • For each halogen subtract 1 H Cl 1,3-dichloro cycloctane C8H14Cl2 Cl Practice F 2 fluoro 1 butene C4H7F Br Br 2,5-dibromo 3-ethyl 4-methyl heptane C10H20Br2 Alcohols • Hydrocarbons with an –OH attached • To name it, give it the suffix –(an)ol and the number the OH is attached to • Normally you subtract one H from the main group and put an OH on the end (to signify it is an alcohol) H O C2H5OH Ethanol OH C3H7OH 2 propanol Commonly Isopropanol or Rubbing alcohol Aldehydes • Hydrocarbons with a =O on the outer edge of the chain • (most have a foul stench, like formaldehyde or methanal) • To name it add the suffix “–al” • For the formula subtract 2 H and add O =O O= hexanal C6H12O octanal C8H16O Ketones • Hydrocarbons with a =O not on the edge of the compound • To name it add the suffix “–one” • For the formula subtract 2 H and add O O= O= cyclopropanone C3H4O 3-nonanone C9H18O Carboxylic Acid • Hydrocarbons with a –COOH group attached • To name it give it the suffix “–oic acid”, the C in the group does count • Subtract one C one H and add COOH • This group looks like… R-C=O O H Pentanoic acid C4H9COOH =O O H Everything so far… • Alkanes, alkenes, and alkynes • Isomers, halogenated and cyclic -OH *R means any carbon chain Alcohols Carboxylic Acids R-OH R-C=O -ol -oic acid -al Ketones R-C-R =O Aldehydes on the end R=O -one Predicting organic reactions • Addition reactions occur by adding halogens or hydrogen to alkene or alkynes. • In the reaction, the new molecule takes the place of the double or triple bond. • Cl2 + CH3-CH=CH2 CH3-CClH- CClH2 example • 1- butene is reacted with fluorine • C4 H8 + F2 C4H8F2 Predicting organic reactions • Substitution reactions occur by adding halogens to an alkane. • In the reaction, the new molecule takes the place of a hydrogen. • Cl2 + CH3-CH3 CH3-CClH2 + HCl • Cl2 + C2H6 C2ClH5 + HCl Predicting organic reactions • Combustion reactions occur when an organic compound is burned in oxygen. • The products of a complete combustion are water vapor and carbon dioxide. • C6H12O6 + 6 O2 6 H2O+ 6 CO2 Predicting organic reactions • Esterification reactions • Made by reacting carboxylic acids with alcohols. Carboxylic acid + H-O-R R-C-O-R alcohol O= O= R-C-O-H Ester + H-O-H Examples • Fluorine is added to 1 propene • Ethanol is burned in oxygen • Chlorine is added to propane • Ethanoic acid is reacted with 1-butanol What is petroleum? • Also known as crude oil • It is a thick black sludge • It comes from ancient plant and animal life long since buried and kept under extreme pressure for millions of years. • It is composed of countless different organic compounds. What is made from petroleum • Gasoline, kerosene, and rocket fuel • Most plastics and other polymers (elastomers and fibers) • Synthetic rubbers and fabrics • Most pharmaceutical drugs • And several other things • If we run out of petroleum it would have a devastating effect on us One compound that comes from petroleum Benzene Which has the resonance structure It also is drawn as Compounds that contain benzene are called aromatic • Aspirin (acetyl salicylic acid) O-H O O= O Compounds that contain benzene are called aromatic • Trinitro Toluene (TNT) - NO2 O2N- O2N- A few other aromatics • Vinyl, napthalene (found in moth balls), acetaminophen, penacillin • Benzene is an extremely common organic compound • The fact that the double bonds flip back and forth (called resonance) give it a very stable structure Polymerization Esters O= • Esters- a functional group in the middle of a carbon chain; R-COO-R • It gets the suffix –oate It is very similar to carboxylic acids ~In fact, it is formed by a carboxylic acid and an alcohol R-C-O-R + H-O-R R-C-O-R the water came from… O= O= R-C-O-H + H-O-H Now if you have a few compounds that have both a O= + H-O-H H-O-R-C-O- R-C-O-H O= O= O= Carboxylic acid end +an alcohol end H-O-R-C-O-H H-O-R-C-O-H + They could form an ester that looks like… But the compound still has a… And an alcohol end Carboxylic acid end So it could repeat this process thousands even millions of times and make a whole bunch of… poly esters Of course, the scientific prefix for “whole bunch of” is This is the basis for a polymer • Polymer-A large chain-like molecule composed of smaller molecules linked together • The smaller units it is made up of are called monomers • monomers need to have ends that can join together (or stack on top of one another) • Like an extension cord or markers • So you could (infinitely) join them together to make a large polymer Polymers can get very large • common polymers have a molecular mass of around 50,000 g/mol • The first molecules seen under a microscope were polymer chains • Common polymers include things like… • Nylon, Kevlar©, latex, PVC, rubber, acrylic, vinyl, Deoxyribonucleic acid (DNA) and carbohydrates Piece of DNA Polymers are put into three classes Plastics ElastomersPolymers that can be stretched to 10x their normal size and return to their original shape Elastic Fibers Polymers that cannot stretch or be reshaped once formed Nylon and Acrylic Polymers that can stretch and flex more than fibers but less than Elastomers Polypropylene polystyrene and PVC (polyvinyl chloride)