Alcohols, Phenols, and Ethers
... Lower molecular weight alcohols are soluble in water. This is due to hydrogen bonding between hydroxy group and water. ...
... Lower molecular weight alcohols are soluble in water. This is due to hydrogen bonding between hydroxy group and water. ...
AQA A-level Chemistry
... l If two moles of a fuel are combusted then the standard enthalpy of reaction is the standard enthalpy of combustion multiplied by 2. l If four moles of a compound are formed from its elements in their standard states, then the standard enthalpy of formation value must be multiplied by 4 to get th ...
... l If two moles of a fuel are combusted then the standard enthalpy of reaction is the standard enthalpy of combustion multiplied by 2. l If four moles of a compound are formed from its elements in their standard states, then the standard enthalpy of formation value must be multiplied by 4 to get th ...
study guide spring 2012
... 100. A compound’s empirical formula is N2O5. If the formula mass is 108 amu, its molecular formula is ____________________. 101. A compound’s empirical formula is CH3. If the formula mass is 30 amu, its molecular formula is ____________________. 102. To calculate the percentage composition of NiCl2, ...
... 100. A compound’s empirical formula is N2O5. If the formula mass is 108 amu, its molecular formula is ____________________. 101. A compound’s empirical formula is CH3. If the formula mass is 30 amu, its molecular formula is ____________________. 102. To calculate the percentage composition of NiCl2, ...
NAME - HCC Learning Web
... Trans methyl groups favor chair and both are equatorial. Trans methyl groups favor chair and both are axial. Trans methyl groups favor twist-boat conformation. Trans methyl groups favor chair and are axial and equatorial. ...
... Trans methyl groups favor chair and both are equatorial. Trans methyl groups favor chair and both are axial. Trans methyl groups favor twist-boat conformation. Trans methyl groups favor chair and are axial and equatorial. ...
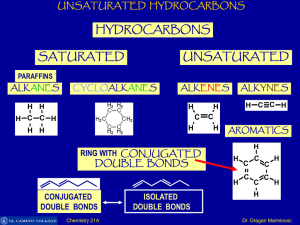
Unsaturated Hydrocarbons
... UNSATURATED HYDROCARBONS All the C-atoms in benzene are sp2-hybridized. Two sp2-hybrid orbitals of each C-atom overlap with two sp2-hybrid orbital of two other C-atoms to form sigma bonds. In this way there are six sigma bonds are formed between six C-atoms which are 120o apart. Remaining six sp2-o ...
... UNSATURATED HYDROCARBONS All the C-atoms in benzene are sp2-hybridized. Two sp2-hybrid orbitals of each C-atom overlap with two sp2-hybrid orbital of two other C-atoms to form sigma bonds. In this way there are six sigma bonds are formed between six C-atoms which are 120o apart. Remaining six sp2-o ...
CH3
... 3. Cite some industrial uses of organic compounds ETHER. References: All organic chemistry book will do ...
... 3. Cite some industrial uses of organic compounds ETHER. References: All organic chemistry book will do ...
O-VOCS - Tubitak Journals
... a reaction product, will not adsorb onto the catalyst, particularly at low reaction temperatures. γ -Al 2 O 3 is commonly used as an adsorbent and a catalyst or catalyst support in many chemical processes, including the cracking, hydrocracking, and hydrodesulfurization of petroleum feed stocks. It i ...
... a reaction product, will not adsorb onto the catalyst, particularly at low reaction temperatures. γ -Al 2 O 3 is commonly used as an adsorbent and a catalyst or catalyst support in many chemical processes, including the cracking, hydrocracking, and hydrodesulfurization of petroleum feed stocks. It i ...
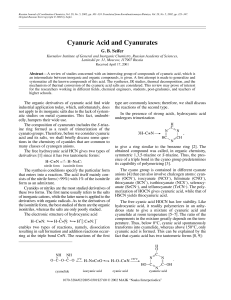
Cyanuric Acid and Cyanurates
... The bromine derivative, which is analogous to cyanuric chloride, is obtained by reacting HBr with cyanogen bromide (BrCN) in benzene or via polymerization of BrCN in the presence of small quantities of Br2 [39]. The bromine atoms in C3N3Br3 are sufficiently mobile, and therefore, this compound can b ...
... The bromine derivative, which is analogous to cyanuric chloride, is obtained by reacting HBr with cyanogen bromide (BrCN) in benzene or via polymerization of BrCN in the presence of small quantities of Br2 [39]. The bromine atoms in C3N3Br3 are sufficiently mobile, and therefore, this compound can b ...
Catalytic hydrogenation
... Noyori has coined the term “metal-ligand bifunctional catalysts, describing systems containing an ancillary ligand cis to the hydride that assists in the hydride transfer step and this ligand must have an NH or OH (protic) group. ...
... Noyori has coined the term “metal-ligand bifunctional catalysts, describing systems containing an ancillary ligand cis to the hydride that assists in the hydride transfer step and this ligand must have an NH or OH (protic) group. ...
The story of V
... These unpaired electrons (on the radicals) will be quite discontent with being alone and still want to be paired. If they can find ANY electron to pair up with, they will do so. The carbon-carbon double bond in a vinyl monomer, or in a DVDE, has a pair of electrons which is very easily attacked by t ...
... These unpaired electrons (on the radicals) will be quite discontent with being alone and still want to be paired. If they can find ANY electron to pair up with, they will do so. The carbon-carbon double bond in a vinyl monomer, or in a DVDE, has a pair of electrons which is very easily attacked by t ...
Soln Chem 2008Nov(9746)
... Boiling point increases from C2H5Cl to C2H5I due to stronger intermolecular van der Waals' forces as the number of electrons increases from C2H5Cl to C2H5I. (ans) (ii) bond polarity: C–Cl > C–Br > C–I Bond polarity decreases from C–Cl to C–I due to decrease in electronegativity from Cl to I. (ans) ...
... Boiling point increases from C2H5Cl to C2H5I due to stronger intermolecular van der Waals' forces as the number of electrons increases from C2H5Cl to C2H5I. (ans) (ii) bond polarity: C–Cl > C–Br > C–I Bond polarity decreases from C–Cl to C–I due to decrease in electronegativity from Cl to I. (ans) ...
Silylation Overview - Sigma
... always a necessity for up-to-date reviews. The silylation of organic compounds for synthetic and analytical purposes, an important part of organosilicon chemistry, is the subject matter of this totally revised and enlarged monograph. The term “silylation” is defined as the substitution of a hydrogen ...
... always a necessity for up-to-date reviews. The silylation of organic compounds for synthetic and analytical purposes, an important part of organosilicon chemistry, is the subject matter of this totally revised and enlarged monograph. The term “silylation” is defined as the substitution of a hydrogen ...
direct synthesis of hydrogen peroxide from oxygen and hydrogen
... Figure 2 Chemical principle of the AO process........................................................................... 10 Figure 3 Schematic diagram of the AO process .......................................................................... 11 Figure 4 Flow sheet of a typical AO process for the p ...
... Figure 2 Chemical principle of the AO process........................................................................... 10 Figure 3 Schematic diagram of the AO process .......................................................................... 11 Figure 4 Flow sheet of a typical AO process for the p ...
IUPAC Provisional Recommendations
... Inorganic Chemistry (see ref. 14). In the nuclide symbol, the atomic symbol is printed in roman type, italicized atomic symbols being reserved for letter locants, as is customary in the nomenclature of organic compounds and described in P-14.3. For the hydrogen isotopes protium, deuterium and tritiu ...
... Inorganic Chemistry (see ref. 14). In the nuclide symbol, the atomic symbol is printed in roman type, italicized atomic symbols being reserved for letter locants, as is customary in the nomenclature of organic compounds and described in P-14.3. For the hydrogen isotopes protium, deuterium and tritiu ...
The Acidic Environment #2
... Multi‐protic acids go through stepwise ionisation, from which it can be seen that complex ions can be made, eg hydrogen carbonate (HCO3–) When a strong acid and weak base react to form a salt, the anion conjugate acid of the weak base acts as an acid (while the acid’s conjugate is too weak) and t ...
... Multi‐protic acids go through stepwise ionisation, from which it can be seen that complex ions can be made, eg hydrogen carbonate (HCO3–) When a strong acid and weak base react to form a salt, the anion conjugate acid of the weak base acts as an acid (while the acid’s conjugate is too weak) and t ...
Ketones and Aldehydes Reading: Wade chapter 18, sections 18
... dipole moment attributable to the carbonyl group, giving ketones and aldehydes higher boiling points than hydrocarbons. However, the dipole-dipole interaction is weaker than hydrogen-bonding interactions, and thus ketones and aldehydes have lower boiling points than alcohols. Nevertheless, aldehydes ...
... dipole moment attributable to the carbonyl group, giving ketones and aldehydes higher boiling points than hydrocarbons. However, the dipole-dipole interaction is weaker than hydrogen-bonding interactions, and thus ketones and aldehydes have lower boiling points than alcohols. Nevertheless, aldehydes ...
File
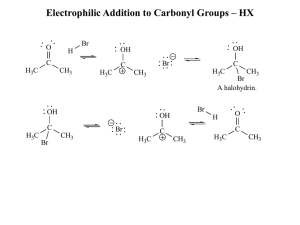
... A carbonyl group is a carbon atom double bonded to an oxygen atom. Aldehyde: carbon of the carbonyl group is always joined to at least one hydrogen (RCHO); ketone: carbon is joined to two other carbons (RCOR). Carboxylic acid: carbon is attached to hydroxyl group (RCOOH). Ester: a derivative of a ca ...
... A carbonyl group is a carbon atom double bonded to an oxygen atom. Aldehyde: carbon of the carbonyl group is always joined to at least one hydrogen (RCHO); ketone: carbon is joined to two other carbons (RCOR). Carboxylic acid: carbon is attached to hydroxyl group (RCOOH). Ester: a derivative of a ca ...
Document
... group as the parent alkane and number it from the end that gives the -OH the lower number 2.change the ending of the parent alkane from -e to -ol and use a number to show the location of the -OH group; for cyclic alcohols, the carbon bearing the -OH group is carbon-1 3.name and number substituents a ...
... group as the parent alkane and number it from the end that gives the -OH the lower number 2.change the ending of the parent alkane from -e to -ol and use a number to show the location of the -OH group; for cyclic alcohols, the carbon bearing the -OH group is carbon-1 3.name and number substituents a ...
Document
... • Though the simplest phosphazene superbase, P1-Me, was first synthesized in 1975, chemists assumed that the compounds were highly unstable, like their alkyl-substituted derivatives. The species was regarded at that time as little more than an academic curiosity. ...
... • Though the simplest phosphazene superbase, P1-Me, was first synthesized in 1975, chemists assumed that the compounds were highly unstable, like their alkyl-substituted derivatives. The species was regarded at that time as little more than an academic curiosity. ...
Chem. Soc. Rev., 2015, 44, 2202--2220 - RSC Publishing
... a competing reaction pathway must be added to the established mechanistic picture. These intermediates emerge from the abstraction of a proton in the a-position of the silyliminium ion in VI (VI VIII and IX). The resulting iminium ion in IX is reduced to the free amine X (IX - X). That free amine X ...
... a competing reaction pathway must be added to the established mechanistic picture. These intermediates emerge from the abstraction of a proton in the a-position of the silyliminium ion in VI (VI VIII and IX). The resulting iminium ion in IX is reduced to the free amine X (IX - X). That free amine X ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.