chapter 22 organic and biological molecules
... a. A polyester forms when an alcohol functional group reacts with a carboxylic acid functional group. The monomer for a homopolymer polyester must have an alcohol functional group and a carboxylic acid functional group present in the structure. b. A polyamide forms when an amine functional group rea ...
... a. A polyester forms when an alcohol functional group reacts with a carboxylic acid functional group. The monomer for a homopolymer polyester must have an alcohol functional group and a carboxylic acid functional group present in the structure. b. A polyamide forms when an amine functional group rea ...
Chem. Soc. Rev., 2015, 44, 2202--2220 - RSC Publishing
... a competing reaction pathway must be added to the established mechanistic picture. These intermediates emerge from the abstraction of a proton in the a-position of the silyliminium ion in VI (VI VIII and IX). The resulting iminium ion in IX is reduced to the free amine X (IX - X). That free amine X ...
... a competing reaction pathway must be added to the established mechanistic picture. These intermediates emerge from the abstraction of a proton in the a-position of the silyliminium ion in VI (VI VIII and IX). The resulting iminium ion in IX is reduced to the free amine X (IX - X). That free amine X ...
Thermochemistry - hrsbstaff.ednet.ns.ca
... universe is constant. In other words, energy can be neither destroyed nor created. This idea can be expressed by the following equation: ∆Euniverse = 0 Energy can, however, be transferred from one substance to another. It can also be converted into various forms. In order to interpret energy changes ...
... universe is constant. In other words, energy can be neither destroyed nor created. This idea can be expressed by the following equation: ∆Euniverse = 0 Energy can, however, be transferred from one substance to another. It can also be converted into various forms. In order to interpret energy changes ...
acetyl coenzyme A - Fakultas Farmasi Unand
... Dimethylallyl pyrophosphate has a leaving group (pyrophosphate) at an allylic carbon; it is reactive toward nucleophilic substitution at this position. Dr. Wolf's CHM 424 ...
... Dimethylallyl pyrophosphate has a leaving group (pyrophosphate) at an allylic carbon; it is reactive toward nucleophilic substitution at this position. Dr. Wolf's CHM 424 ...
- University at Albany
... Similar to that of alkenes, except the ending of the root name corresponding to the longest continuous chain containing the triple bond is changed from “ene” to “yne”. E.g. ethene becomes ethyne, propene becomes propyne. The chain is numbered from the end closest to the triple bond. When a double an ...
... Similar to that of alkenes, except the ending of the root name corresponding to the longest continuous chain containing the triple bond is changed from “ene” to “yne”. E.g. ethene becomes ethyne, propene becomes propyne. The chain is numbered from the end closest to the triple bond. When a double an ...
Chemistry Final Exam Review
... ____ 24. A reversible chemical reaction means that the reaction can travel forwards or backwards. ____ 25. Subscripts are used to balance chemical reactions. ____ 26. A synthesis reaction contains two products. ____ 27. A decomposition reaction contains at least two products. ____ 28. A combustion r ...
... ____ 24. A reversible chemical reaction means that the reaction can travel forwards or backwards. ____ 25. Subscripts are used to balance chemical reactions. ____ 26. A synthesis reaction contains two products. ____ 27. A decomposition reaction contains at least two products. ____ 28. A combustion r ...
Hydrocarbons
... hydrocarbon that contains one or more benzene-like rings –arene: a term used to describe aromatic compounds –Ar-: a symbol for an aromatic group derived by removing an -H from an arene ...
... hydrocarbon that contains one or more benzene-like rings –arene: a term used to describe aromatic compounds –Ar-: a symbol for an aromatic group derived by removing an -H from an arene ...
General and Inorganic Chemistry – Laboratory Techniques
... substance is a homogeneous material consisting of one particular kind of matter. A mixture is a material that can be separated by physical means into two or more substances. A substance is a kind of matter that cannot be separated into other kinds of matter by any physical process. Substances can be ...
... substance is a homogeneous material consisting of one particular kind of matter. A mixture is a material that can be separated by physical means into two or more substances. A substance is a kind of matter that cannot be separated into other kinds of matter by any physical process. Substances can be ...
Alcohols, Phenols, Thiols and Ethers
... When a secondary alcohol is oxidized [O], One H is removed from the –OH. Another H is removed from the carbon bonded to the OH. A ketone is produced. [O] secondary alcohol ...
... When a secondary alcohol is oxidized [O], One H is removed from the –OH. Another H is removed from the carbon bonded to the OH. A ketone is produced. [O] secondary alcohol ...
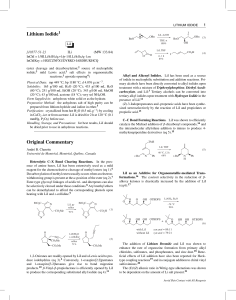
Lithium Iodide Original Commentary - Groupe Charette
... The stereoselective ring opening of an allylic epoxide to an (E)allylic iodide was achieved using a combination of LiI and scandium trifluoromethanesulfonate (eq 12).28 In contrast, 9-bromo9-borabicyclo[3.3.1]nonane furnished the corresponding (Z)allylic bromide selectively. LiI transforms 2,3-epoxy ...
... The stereoselective ring opening of an allylic epoxide to an (E)allylic iodide was achieved using a combination of LiI and scandium trifluoromethanesulfonate (eq 12).28 In contrast, 9-bromo9-borabicyclo[3.3.1]nonane furnished the corresponding (Z)allylic bromide selectively. LiI transforms 2,3-epoxy ...
Chapter 13
... Solubility of Alcohols and Ethers in Water Alcohols and ethers are more soluble in water than alkanes because the oxygen atom hydrogen bonds with water. Alcohols with 1-4 C atoms are soluble, but alcohols with 5 or more C atoms are not. ...
... Solubility of Alcohols and Ethers in Water Alcohols and ethers are more soluble in water than alkanes because the oxygen atom hydrogen bonds with water. Alcohols with 1-4 C atoms are soluble, but alcohols with 5 or more C atoms are not. ...
evaluation copy
... material for any class he or she teaches. No part of these activities may be used or reproduced in any other manner without prior written permission of PASCO scientific, except in the case of brief quotations used in critical articles or reviews. SPARK Science Learning System, SPARKvue, PASCO Capsto ...
... material for any class he or she teaches. No part of these activities may be used or reproduced in any other manner without prior written permission of PASCO scientific, except in the case of brief quotations used in critical articles or reviews. SPARK Science Learning System, SPARKvue, PASCO Capsto ...
File
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
Chapter 11 Unsaturated Hydrocarbons
... • the reaction occurs without a catalyst • a dihaloalkane product results In the general equation for halogenation, X2 is used for either Cl2 or Br2. ...
... • the reaction occurs without a catalyst • a dihaloalkane product results In the general equation for halogenation, X2 is used for either Cl2 or Br2. ...
THE FRIEDEL-CRAFTS BENZYLATION OF ARENES AND THE
... First, I would like to thank my supervisor Prof. Dr. Jeffrey W. Bode for giving me the opportunity to work in his research group. After the group arrived from UPenn, I was one of his first new PhD students at ETH and I feel really fortunate that I could join the group during this exciting time. Jeff ...
... First, I would like to thank my supervisor Prof. Dr. Jeffrey W. Bode for giving me the opportunity to work in his research group. After the group arrived from UPenn, I was one of his first new PhD students at ETH and I feel really fortunate that I could join the group during this exciting time. Jeff ...
Organic Chemistry Fifth Edition
... Dimethylallyl diphosphate has a leaving group (diphosphate) at an allylic carbon; it is reactive toward nucleophilic substitution at this position. ...
... Dimethylallyl diphosphate has a leaving group (diphosphate) at an allylic carbon; it is reactive toward nucleophilic substitution at this position. ...
AS Chemistry Teacher Handbook
... Common compounds include the acids HCl, HNO 3 , H 2 SO 4 , CH 3 CO 2 H, salts of these acids, Group 1 and Group 2 carbonates, hydroxides, oxides and gases such as CH 4 , CO 2 , NH 3 , SO 2 . Common ions are those from Groups 1, 2, 6 and 7 elements and compound ions such as NH 4 +, NO 3 ‒, OH‒, CO 3 ...
... Common compounds include the acids HCl, HNO 3 , H 2 SO 4 , CH 3 CO 2 H, salts of these acids, Group 1 and Group 2 carbonates, hydroxides, oxides and gases such as CH 4 , CO 2 , NH 3 , SO 2 . Common ions are those from Groups 1, 2, 6 and 7 elements and compound ions such as NH 4 +, NO 3 ‒, OH‒, CO 3 ...
Chapter Three
... Condensation reactions: Condensation reactions are reactions in which two molecules combine into one, with the expulsion of a small molecule such as water. The dehydration of alcohols to form ethers is a condensation reaction. RCH2OH ...
... Condensation reactions: Condensation reactions are reactions in which two molecules combine into one, with the expulsion of a small molecule such as water. The dehydration of alcohols to form ethers is a condensation reaction. RCH2OH ...
Chemical Quantities
... To understand the molecular and mass information given in a balanced equation. Reactions are what chemistry is really all about. Recall from Chapter 6 that chemical changes are actually rearrangements of atom groupings that can be described by chemical equations. These chemical equations tell us the ...
... To understand the molecular and mass information given in a balanced equation. Reactions are what chemistry is really all about. Recall from Chapter 6 that chemical changes are actually rearrangements of atom groupings that can be described by chemical equations. These chemical equations tell us the ...
Unit 8 Chemical Equilibrium Focusing on Acid
... Equilibrium describes any condition or situation of balance. We recognize equilibrium in a chemical reaction system, oddly enough, by noticing nothing—we see no change in any property of the system. The easiest conclusion to draw would be that nothing is happening, but closer study reveals that, at ...
... Equilibrium describes any condition or situation of balance. We recognize equilibrium in a chemical reaction system, oddly enough, by noticing nothing—we see no change in any property of the system. The easiest conclusion to draw would be that nothing is happening, but closer study reveals that, at ...
Chemistry - Department of Education and Skills
... The individual modules making up this handbook have been selected around the content and structure of the syllabus to provide easy access to resource material in a way which supports the implementation of the course. However, it is important to realise that these modules do not define the syllabus. ...
... The individual modules making up this handbook have been selected around the content and structure of the syllabus to provide easy access to resource material in a way which supports the implementation of the course. However, it is important to realise that these modules do not define the syllabus. ...
Lecture Notes Chem 51B S. King Chapter 9 Alcohols, Ethers, and
... VII. Other Methods for Converting Alcohols into Alkyl Halides We have seen that alcohols can be converted to alkyl halides by treating them with HCl, HBr, or HI. Better yields can be obtained , and carbocation rearrangements can be avoided if a phosphorus trihalide (PCl3, PBr3, or PI3), or thionyl ...
... VII. Other Methods for Converting Alcohols into Alkyl Halides We have seen that alcohols can be converted to alkyl halides by treating them with HCl, HBr, or HI. Better yields can be obtained , and carbocation rearrangements can be avoided if a phosphorus trihalide (PCl3, PBr3, or PI3), or thionyl ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.