File
... 1-The only difference between the structures of acetic acid and ethanol is the replacement of a CH2 group (in ethanol) by a carbonyl group (in acetic acid). But carbonyl carbon atom carries a substantial positive charge (δ+). This charge makes it much easier to place a negative charge on the adjacen ...
... 1-The only difference between the structures of acetic acid and ethanol is the replacement of a CH2 group (in ethanol) by a carbonyl group (in acetic acid). But carbonyl carbon atom carries a substantial positive charge (δ+). This charge makes it much easier to place a negative charge on the adjacen ...
Full-Text PDF
... and amines, and thus becoming a rare example of metallasilsesquioxanes performing homogeneous catalysis. Benzene, cyclohexane, and other alkanes, as well as alcohols, can be oxidized in acetonitrile solution to phenol—the corresponding alkyl hydroperoxides and ketones, respectively—by hydrogen perox ...
... and amines, and thus becoming a rare example of metallasilsesquioxanes performing homogeneous catalysis. Benzene, cyclohexane, and other alkanes, as well as alcohols, can be oxidized in acetonitrile solution to phenol—the corresponding alkyl hydroperoxides and ketones, respectively—by hydrogen perox ...
Introduction to Organic Molecules
... • Common examples include alkyl halides, alcohols, ethers, and ...
... • Common examples include alkyl halides, alcohols, ethers, and ...
Application of IBX Method for the Synthesis of Ketones from
... changed into its chloride. Then the acid chloride reacts with an organometallic reagent or gives a FriedelCrafts type reaction in the presence of Lewis acids. These methods are very useful for most aliphatic acid halides and good yields can be obtained in this way. However, if this method is used fo ...
... changed into its chloride. Then the acid chloride reacts with an organometallic reagent or gives a FriedelCrafts type reaction in the presence of Lewis acids. These methods are very useful for most aliphatic acid halides and good yields can be obtained in this way. However, if this method is used fo ...
Chapter 24 Organic Chemistry
... hydrolysis of an ester to yield an acid and an alcohol substitution of an aromatic ring using a halogen oxidation of an alcohol to yield a carboxylic acid neutralization of an amine using a strong mineral acid ...
... hydrolysis of an ester to yield an acid and an alcohol substitution of an aromatic ring using a halogen oxidation of an alcohol to yield a carboxylic acid neutralization of an amine using a strong mineral acid ...
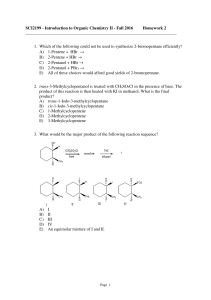
SCI2199 - Introduction to Organic Chemistry II
... A) an SN1-type reaction involving the protonated alcohol as the substrate. B) an SN2-type reaction involving the protonated alcohol as the substrate. C) an E1-type reaction involving the protonated alcohol as the substrate. D) an E2-type reaction involving the protonated alcohol as the substrate. E) ...
... A) an SN1-type reaction involving the protonated alcohol as the substrate. B) an SN2-type reaction involving the protonated alcohol as the substrate. C) an E1-type reaction involving the protonated alcohol as the substrate. D) an E2-type reaction involving the protonated alcohol as the substrate. E) ...
Catalytic, Thermal, Regioselective Functionalization of Alkanes and
... The conversion of alkanes to functionalized products with terminal regioselectivity must confront several challenges outlined in Scheme 1. The internal C-H bond of an alkane is weaker than the terminal C-H bond,(5) and so reactions that are selective for one position over the other are typically sel ...
... The conversion of alkanes to functionalized products with terminal regioselectivity must confront several challenges outlined in Scheme 1. The internal C-H bond of an alkane is weaker than the terminal C-H bond,(5) and so reactions that are selective for one position over the other are typically sel ...
Biomimetic oxidation of catechol employing complexes formed in
... Reaction of catechol oxidation in the presence of copper complexes formed with ligands L1–L7 In order to investigate the ability of the copper complexes formed in-situ to act as catalysts for catecholase-like activity, the catalytic oxidation of the substrate catechol by the a combination of ligands ...
... Reaction of catechol oxidation in the presence of copper complexes formed with ligands L1–L7 In order to investigate the ability of the copper complexes formed in-situ to act as catalysts for catecholase-like activity, the catalytic oxidation of the substrate catechol by the a combination of ligands ...
Ch 8 Lecture 1
... e) Organic molecules with polar functional groups (-OH, -NH2, -CO2H) have much higher water solubilities 2) The longer the alkyl group, the less water soluble the alcohol (more soluble in hydrocarbons—like dissolves like) 3) MeOH and EtOH are very similar to water as solvents: many salts will dissol ...
... e) Organic molecules with polar functional groups (-OH, -NH2, -CO2H) have much higher water solubilities 2) The longer the alkyl group, the less water soluble the alcohol (more soluble in hydrocarbons—like dissolves like) 3) MeOH and EtOH are very similar to water as solvents: many salts will dissol ...
Section D19: Alkanes, Alkenes and Alcohols
... and incomplete combustion of alkanes 3.5 describe the substitution reaction of methane with bromine to form bromomethane in the presence of UV light. 3.6 recall that alkenes have the general formula CnH2n 3.7 draw displayed formulae for alkenes with up to four carbon atoms in a molecule, and name th ...
... and incomplete combustion of alkanes 3.5 describe the substitution reaction of methane with bromine to form bromomethane in the presence of UV light. 3.6 recall that alkenes have the general formula CnH2n 3.7 draw displayed formulae for alkenes with up to four carbon atoms in a molecule, and name th ...
CBSE/12th Class/2010/CHEMISTRY
... (iii) 1- Bromo-2-methylbutane will undergo SN2 reaction faster than 2- Bromo-2methylbutane because 1- Bromo-2-methylbutane has less steric hindrance than 2Bromo-2-methylbutane. This is because 1- Bromo-2-methylbutane is a primary alkyl halidewhereas 2- Bromo-2-methylbutane is a tertiary alkyl halide ...
... (iii) 1- Bromo-2-methylbutane will undergo SN2 reaction faster than 2- Bromo-2methylbutane because 1- Bromo-2-methylbutane has less steric hindrance than 2Bromo-2-methylbutane. This is because 1- Bromo-2-methylbutane is a primary alkyl halidewhereas 2- Bromo-2-methylbutane is a tertiary alkyl halide ...
Isomerism of coordination compounds
... Isomerism of coordination compounds Structural isomerism Ionization isomerism When ligands are exchanged in a complex by the counter-ion, it is called ionization isomerism. e.g. [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl Linkage isomerism A ligand connects with the central metal atom through different ato ...
... Isomerism of coordination compounds Structural isomerism Ionization isomerism When ligands are exchanged in a complex by the counter-ion, it is called ionization isomerism. e.g. [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl Linkage isomerism A ligand connects with the central metal atom through different ato ...
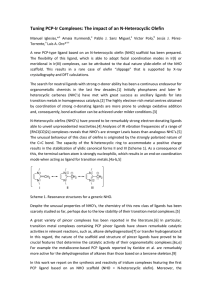
Tuning PCP-Ir Complexes: The impact of an N
... A new PCP-type ligand based on an N-heterocyclic olefin (NHO) scaffold has been prepared. The flexibility of this ligand, which is able to adopt facial coordination modes in Ir(I) or meridional in Ir(III) complexes, can be attributed to the dual nature ylide-olefin of the NHO scaffold. This results ...
... A new PCP-type ligand based on an N-heterocyclic olefin (NHO) scaffold has been prepared. The flexibility of this ligand, which is able to adopt facial coordination modes in Ir(I) or meridional in Ir(III) complexes, can be attributed to the dual nature ylide-olefin of the NHO scaffold. This results ...
Table 5 - Utrecht University Repository
... The CNF are of the fishbone type, i.e., with the graphite planes oriented at an angle to the central axis and the fibers interwoven into porous bodies. After removal of the growth catalyst and the activation treatment with nitric acid for 2 h the nonmicroporous fibers exhibited a specific surface ar ...
... The CNF are of the fishbone type, i.e., with the graphite planes oriented at an angle to the central axis and the fibers interwoven into porous bodies. After removal of the growth catalyst and the activation treatment with nitric acid for 2 h the nonmicroporous fibers exhibited a specific surface ar ...
PREPARATION OF ORGANOLITHIUM COMPOUNDS - GCG-42
... INTRODUCTION Organometallic compounds are the Compounds containing a carbon –metal bond . Since lithium is a metal , so it forms an organo-metallic compound. The compounds formed by carbon– lithium bonds are called organolithium compounds ...
... INTRODUCTION Organometallic compounds are the Compounds containing a carbon –metal bond . Since lithium is a metal , so it forms an organo-metallic compound. The compounds formed by carbon– lithium bonds are called organolithium compounds ...
Linear Functionalized Polyethylene Prepared with Highly Active Neutral Ni(II) Complexes
... important when the polymer of interest is sparingly soluble in the solvent blend. When the integrations did not agree with each other via WALTZ, an inverse-gated experiment was run to check if some of the signals were being selectively enhanced. Little difference in the integration was observed in m ...
... important when the polymer of interest is sparingly soluble in the solvent blend. When the integrations did not agree with each other via WALTZ, an inverse-gated experiment was run to check if some of the signals were being selectively enhanced. Little difference in the integration was observed in m ...
Exam 2-07
... c.) they have more hydrogen atoms d.) the cis double bonds give them an irregular shape e.) they have fewer hydrogen atoms ...
... c.) they have more hydrogen atoms d.) the cis double bonds give them an irregular shape e.) they have fewer hydrogen atoms ...
T919 Oxidation and Reduction Past Paper Questions
... Both zinc and tin are used to coat iron to prevent it from rusting. Once the surface is scratched, oxygen and water containing dissolved ions come into contact with the iron and the coating metal. ...
... Both zinc and tin are used to coat iron to prevent it from rusting. Once the surface is scratched, oxygen and water containing dissolved ions come into contact with the iron and the coating metal. ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.